GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
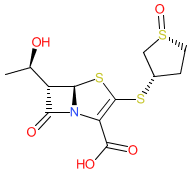
Synonyms: CP-70,429 | CP-70429 | CP70429 | Orlynvah® (sulopenem etzadroxil and probenecid)
sulopenem is an approved drug (FDA (2024))
Compound class:
Synthetic organic
Comment: Sulopenem is a β-lactam (carbapenem) antibacterial [4]. It has a broad-spectrum of activity against Gram-negative, Gram-positive and anaerobic bacteria, including multi-drug resistant Gram-negative infections in both the hospital and community settings. Sulopenem is relatively stable against hydrolysis by various β-lactamases. It is being developed for oral and parenteral administration.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Oral and i.v. sulopenem are being evaluated in three pivotal Phase 3 clinical trials of uncomplicated urinary tract infections, complicated urinary tract infections and complicated intra-abdominal infections (cIAI). All of these trials have been completed. Updates released online (in late 2019) indicate that the drug narrowly missed its primary endpoint in the cIAI study [6]. Results from the UTI studies have been posted with ClinicalTrials.gov. The FDA approved use of sulopenem etzadroxil and probenecid (a renal tubular transport inhibitor) in October 2024, to treat women with uncomplicated urinary tract infections caused by certain susceptible microorganisms (Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis) for which there are limited/no alternative oral antibacterial treatments available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03354598 | Oral Sulopenem-etzadroxil/Probenecid Versus Ciprofloxacin for Uncomplicated Urinary Tract Infection in Adult Women | Phase 3 Interventional | Iterum Therapeutics, International Limited | ||
| NCT03357614 | Sulopenem Followed by Sulopenem-etzadroxil/Probenecid vs Ertapenem Followed by Cipro for Complicated UTI in Adults | Phase 3 Interventional | Iterum Therapeutics, International Limited | 1-2 | |
| NCT03358576 | Sulopenem Versus Ertapenem for Complicated Intra-abdominal Infection (cIAI) | Phase 3 Interventional | Iterum Therapeutics, International Limited | ||