GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
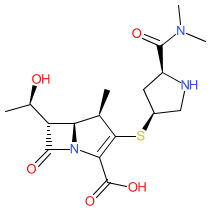
Synonyms: ICI 194,660 | Merrem® | SM-7338
meropenem is an approved drug (FDA (1996), EMA (2018))
Compound class:
Synthetic organic
Comment: Meropenem is a parenterally delivered, broad-spectrum carbapenem antibacterial. It is active against Gram-positive and Gram-negative bacteria, and is effective against aerobic and anaerobic pathogens including Klebsiella, E. coli, Enterococcus, Clostridium spp.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| The FDA granted approval for meropenem as a monotherapy in 1996; indicated for complicated infections of the skin or stomach and bacterial meningitis. In the EU, meropenem is approved for use in fixed-dose combination with vaborbactam (Vaborem®, previosly called Vabomere). Vaborem was authorised by the EMA in 2018, and was indicated for complicated urinary tract (and kidney) infections, complicated intra-abdominal infections, and hospital-acquired and ventilator-associated pneumonias. |
External links  |
|
For extended ADME data see the following: Drugs.com European Medicines Agency (EMA) |