GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
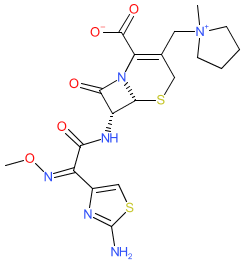
Synonyms: BMY-28142 | BMY28142 | CFPM | Maxipime® | Renapime®
cefepime is an approved drug (FDA (1996), UK (2017))
Compound class:
Synthetic organic
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Cefepime is reserved for the treatment of severe nosocomial pneumonia, particularly infections caused by multi-resistant microorganisms such as Pseudomonas aeruginosa. It does not have a Europe-wide EMA authorisation but is approved by most of the individual nations. Cefepime + taniborbactam (formerly VNRX-5133, an injectable beta-lactamase inhibitor with in vitro activity against both serine- and metallo-beta-lactamases) is proposed as an option for infections due to carbapenem-resistant Enterobacteriaceae and Pseudomonas aeruginosa, and to combat engineerable bioterror pathogens such as Burkholderia species. This combination has completed Phase 3 evaluation for efficacy in the treatment of complicated urinary tract infections (cUTIs; NCT03840148) [3,5]. Cefepime + enmetazobactam has also been evaluated for the treatment of cUTIs [2]. The combination (Exblifep®) was FDA approved for this indication in February 2024 and by the EMA the following month. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03840148 | Safety and Efficacy Study of Cefepime/VNRX-5133 in Patients With Complicated Urinary Tract Infections (CERTAIN-1) | Phase 3 Interventional | VenatoRx Pharmaceuticals, Inc. | A study examining the combination of the 4th gen cephalosporin cefepime and the broad-spectrum β-lactamse inhibitor taniborbactam. | 3,5 |
| NCT03687255 | Safety and Efficacy Study of Cefepime-AAI101 in the Treatment of Complicated Urinary Tract Infections | Phase 3 Interventional | Allecra | 2 | |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com |