GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
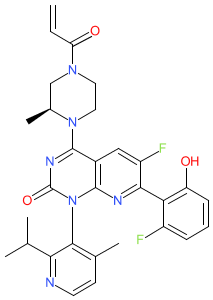
Synonyms: AMG 510 | AMG-510 | AMG510 | compound (R)-38 [PMID: 31820981] | Lumakras® | Lumykras®
sotorasib is an approved drug (FDA (2021), EMA (2022))
Compound class:
Synthetic organic
Comment: Sotorasib (AMG510) is a covalent KRASG12C inhibitor [2] that was developed by Amgen for anti-tumour potential in KRASG12C-driven cancers. It was the first KRAS-targeting drug to be approved for clinical use.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Sotorasib (AMG510) progressed to Phase 3 clinical evaluation in KRASG12C positive NSCLC. The FDA had granted AMG510 breakthrough-therapy designation for locally advanced or metastatic, pretreated KRASG12C positive NSCLC in 2019. Encouraging Phase 1/2 results were published in late 2020 [1]. Full FDA approval was granted in May 2021, for the indication of KRASG12C-mutated locally advanced or metastatic NSCLC that has been treated with at least one prior systemic therapy. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04303780 | Study to Compare AMG 510 "Proposed INN Sotorasib" With Docetaxel in Non Small Cell Lung Cancer (NSCLC) (CodeBreak 200). | Phase 3 Interventional | Amgen | ||
| NCT03600883 | A Phase 1/2, Study Evaluating the Safety, Tolerability, PK, and Efficacy of AMG 510 in Subjects With Solid Tumors With a Specific KRAS Mutation (CodeBreak 100) | Phase 1/Phase 2 Interventional | Amgen | In this study, encouraging antitumour activity was demonstrated in heavily pretreated advanced KRASG12C-mutated solid cancers. | 1 |