GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: AG-881 | AG881 | Voranigo®
vorasidenib is an approved drug (FDA (2024))
Compound class:
Synthetic organic
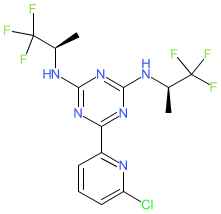
Comment: Vorasidenib (AG-881) inhibits mutant forms of the isocitrate dehydrogenases (IDH1 and IDH2) [4] that catalyse the generation of the oncogenic metabolite d-2-hydroxyglutarate (2-HG). This compound and others, and their use for treating cancer are claimed in patent US10028961 [5]. Vorasidenib can cross the blood-brain barrier, and is being proposed as a chemotherapeutic for IDH mutation +ve cancers including gliomas [3,9] and hematological malignancies [1]. X-ray cocrystal structures of vorasidenib with mutant IDH1 and mutant IDH2 have been deposited with the RCSB Protein Data Bank [4,6]. The X-ray structures show that vorasidenib binds at an allosteric pocket formed in IDH homodimers, at the interface of the two monomers.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Vorasidenib (AG-881) was developed by Agios Pharmaceuticals and was progressed to Phase 3 evaluation in patients with low grade IDH mutant +ve glioma, and a Phase 1 trial for advanced hematologic malignancies with IDH1 or 2 mutations was conducted. Vorasidenib was issued orphan drug designation by the EMA in January 2023, as a treatment for glioma. The FDA issued full approval for vorasidenib as a post-surgical therapy for grade 2 astrocytoma or oligodendroglioma with susceptible IDH1 or IDH2 mutations in August 2024 [2]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02492737 | Study of Orally Administered AG-881 in Patients With Advanced Hematologic Malignancies With an IDH1 and/or IDH2 Mutation | Phase 1 Interventional | Agios Pharmaceuticals, Inc. | ||
| NCT04164901 | Study of AG-881 in Participants With Residual or Recurrent Grade 2 Glioma With an IDH1 or IDH2 Mutation (INDIGO) | Phase 3 Interventional | Agios Pharmaceuticals, Inc. | 8 | |
| NCT02481154 | Study of Orally Administered AG-881 in Patients With Advanced Solid Tumors, Including Gliomas, With an IDH1 and/or IDH2 Mutation | Phase 1 Interventional | Servier | 7 | |