GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Hengqu® | SHR-8735
hetrombopag is an approved drug (China (2021))
Compound class:
Synthetic organic
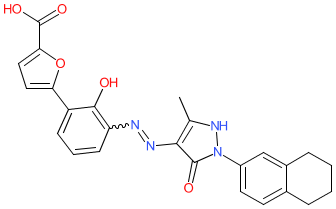
Comment: Hetrombopag is an orally bioavailable, non-peptide small‐molecule thrombopoietin receptor (TPOR/MPL) agonist (TPO-RA), that was developed as a treatment for thrombocytopenia [3]. The parent compound is shown here. Hetrombopag is administered as the ethanolamine salt. Note that the alternative spelling herombopag is used in some publications [5-6].
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Luo F, Luo Z, Luo Y, Zeng H, Chen Y, Cao C. (2025)
Herombopag Versus rhTPO in Stem Cell Transplantation: A Comparative Study of Efficacy, Safety, and Cost-Effectiveness. Transplant Cell Ther, 31 (9): 666.e1-666.e4. [PMID:40588097] |
|
2. Syed YY. (2021)
Hetrombopag: First Approval. Drugs, 81 (13): 1581-1585. [PMID:34357499] |
|
3. Xie C, Zhao H, Bao X, Fu H, Lou L. (2018)
Pharmacological characterization of hetrombopag, a novel orally active human thrombopoietin receptor agonist. J Cell Mol Med, 22 (11): 5367-5377. [PMID:30156363] |
|
4. Yang G, Huang R, Yang S, Zhang X, Yang X, Chen H, Huang Z, Guo C, Pei Q, Tai Y et al.. (2020)
Effect of postdose fasting duration on hetrombopag olamine pharmacokinetics and pharmacodynamics in healthy volunteers. Br J Clin Pharmacol, 86 (8): 1528-1536. [PMID:32069516] |
|
5. Zheng X, Zhang H, Guo W, Cao Y, Liu X, Zhai W, Zhang X, Wang F, Wei J, Yang D et al.. (2023)
Herombopag promotes platelet engraftment and decreases platelet transfusion after allogeneic hematopoietic stem cell transplantation. Eur J Haematol, 110 (5): 527-533. [PMID:36599813] |
|
6. Zhou M, Li T, Zhang P, Lai Y, Sheng L, Ouyang G. (2024)
Herombopag for the treatment of persistent thrombocytopenia following hematopoietic stem cell transplantation. Ann Hematol, 103 (5): 1697-1704. [PMID:38536476] |