GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: example 11 [WO2014151871A9] | TP-0184 | TP0184
Compound class:
Synthetic organic
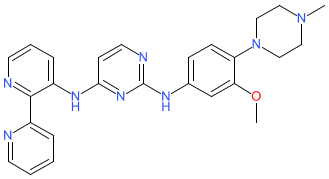
Comment: The chemical structure that was submitted to the WHO for the INN itacnosertib is identical to the structure of example 11 that is claimed in Tolero Pharmaceuticals' patent WO2014151871A9 [1]. The INN submission declares itacnosertib as a 'serine/threonine kinase inhibitor'. Data presented in WO2014151871A9 provide evidence of example 11's activity as an inhibitor of the activin A receptor type 1 (ACVR1; a.k.a. ALK2) which is a Type I receptor serine/threonine kinase. Tolero have declared an ACVR1-targeting oncology programme on their pipeline webpage. The research code provided is TP-0184, but to date (Dec 2020) there has been no formal name>structure disclosure for this compound.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| If itacnosertib/example 11 is indeed TP-0184, the compound has been advanced to Phase 1 evaluation as monotherapy in patients with ACVR1 pathway-activated advanced solid tumours. Click here to link to ClinicalTrials.gov's full list of TP-0184 studies. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03429218 | First-in-human Study of Oral TP-0184 in Patients With Advanced Solid Tumors | Phase 1 Interventional | Sumitomo Dainippon Pharma Oncology, Inc | ||
| NCT04623996 | A Study of TP-0184 to Treat Anemia in Adults With IPSS-R Low or Intermediate Risk MDS | Phase 1/Phase 2 Interventional | Sumitomo Dainippon Pharma Oncology, Inc | ||