GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
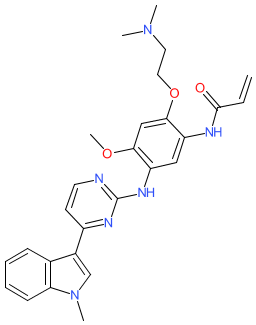
Synonyms: example 1 [WO2016094821A2]
rezivertinib is an approved drug
Compound class:
Synthetic organic
Comment: The chemical structure submitted to the WHO for INN rezivertinib, is identical to the structure of example 1 that is claimed in patent WO2016094821A2 by Beta Pharma [1]. This patent claims novel EGFR inhibitors that are active against mutated EGFRs that are found in cancers, i.e. third generation EGFR inhibitors. From Beta Pharma's declared pipeline, example 1 is likely to be BPI-7711, which is being investigated for the treatment of EGFRT790M-mutant non-small cell lung cancer. We await formal name>structure confirmation. Experimental evidence in WO2016094821A2 indicates that example 1 is orally bioavailable.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| If example 1 is indeed BPI-7711, this inhibitor has been progressed to Phase 3 evaluation in patients with EGFR-mutant NSCLC. Clinical trial NCT03866499 is testing BPI-7711 monotherapy in direct comparison with gefitinib monotherapy. The Chinese drug regulator (NMPA) approved rezivertinib (Rapida®) in 2024, to treat EGFR T790M-positive NSCLC [2]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03866499 | A Study of BPI-7711 Capsule in Non-small Cell Lung Cancer Patients | Phase 3 Interventional | Beta Pharma, Inc. | ||