GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: ALKS-8700 | ALKS8700 | BIIB098 | Vumerity®
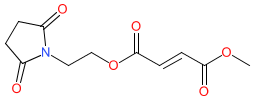
diroximel fumarate is an approved drug (FDA (2019), EMA (2021))
Compound class:
Synthetic organic
Comment: Diroximel fumarate is a novel oral fumarate prodrug. It is rapidly converted to monomethyl fumarate, which is the same active metabolite of dimethyl fumarate.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| FDA approval of diroximel fumarate (October 2019) allows it use as a treatment for relapsing forms of multiple sclerosis, including clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease. Phase 3 study results indicate that diroximel fumarate has improved patient-reported gastrointestinal tolerability compared to dimethyl fumarate (Tecfidera®) [1]. |