GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
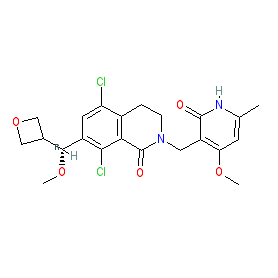
Synonyms: compound 23a [PMID: 29211475] | PF-06821497 | PF06821497
Compound class:
Synthetic organic
Comment: PF-06821497 is an orally active inhibitor of the histone methyltransferase, enhancer of zeste homolog 2 (EZH2), that was developed by Pfizer for antineoplastic potential [1]. Inhibition of EZH2 activity reduces mono-, di-, and trimethylation of lysine 27 on histone H3 (H3K27me, me2, and me3), and this ultimately leads to an antiproliferative effect on susceptible cancer cells (i.e. where aberrant EZH2-mediated epigenetic alterations are driving tumour initiation and progression).
The chemical structure of PF-06821497 matches that for the INN mevrometostat (WHO proposed INN list 131, August 2024). Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Kung PP, Bingham P, Brooun A, Collins M, Deng YL, Dinh D, Fan C, Gajiwala KS, Grantner R, Gukasyan HJ et al.. (2018)
Optimization of Orally Bioavailable Enhancer of Zeste Homolog 2 (EZH2) Inhibitors Using Ligand and Property-Based Design Strategies: Identification of Development Candidate (R)-5,8-Dichloro-7-(methoxy(oxetan-3-yl)methyl)-2-((4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-3,4-dihydroisoquinolin-1(2H)-one (PF-06821497). J Med Chem, 61 (3): 650-665. [PMID:29211475] |
|
2. Yap DB, Chu J, Berg T, Schapira M, Cheng SW, Moradian A, Morin RD, Mungall AJ, Meissner B, Boyle M et al.. (2011)
Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation. Blood, 117 (8): 2451-9. [PMID:21190999] |