GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: compound 3 [PMID:26191358] | ESN-364 | ESN364 | Veoza® | Veozah® | Vezoa®
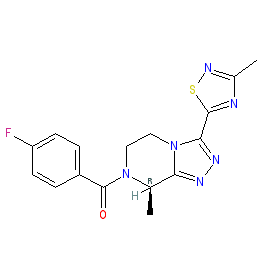
fezolinetant is an approved drug (FDA, EMA & UK MHRA (2023))
Compound class:
Synthetic organic
Comment: Fezolinetant (ESN364) is a potent and selective neurokinin (NK) receptor 3 antagonist [3]. It was developed by Astellas Pharma as a non-hormonal therapeutic option for the treatment of female sex-hormone disorders such as polycystic ovary syndrome [2] and uterine fibroids, and for the management of the vasomotor symptoms (VMS) that drive menopausal hot flashes/flushes and/or night sweats [1,8-9].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Fezolinetant was progressed to Phase 3 clinical evaluation as a non-hormonal therapy for vasomotor symptoms (VMS) due to menopause. Click here to link to ClinicalTrials.gov's full list of fezolinetant studies. The FDA granted clinical approval as a treatment for moderate to severe VMS in May 2023 [6,9]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05033886 | A Study of Fezolinetant to Treat Hot Flashes in Women Going Through Menopause | Phase 3 Interventional | Astellas Pharma Inc | ||
| NCT05034042 | A Study to Find the Best Dose of Fezolinetant to Treat Hot Flashes in Women Going Through Menopause | Phase 2 Interventional | Astellas Pharma Inc | ||
| NCT03192176 | A Dose-ranging Study of the Efficacy of ESN364 in Postmenopausal Women Suffering Vasomotor Symptoms (Hot Flashes) | Phase 2 Interventional | Astellas Pharma Inc | In this study fezolinetant was well-tolerated. It rapidly reduced moderate/severe menopausal vasomotor symptoms. | |
| NCT04003155 | A Study to Find Out if Fezolinetant Helps Reduce Moderate to Severe Hot Flashes in Women Going Through Menopause | Phase 3 Interventional | Astellas Pharma Inc | The SKYLIGHT 1 study. | 5,10 |
| NCT04003389 | A Study to Find Out How Safe Long-term Treatment With Fezolinetant is in Women With Hot Flashes Going Through Menopause | Phase 3 Interventional | Astellas Pharma Inc | The SKYLIGHT 4 study. | 7 |
| NCT04003142 | A Study to Find Out if Fezolinetant Helps Reduce Moderate to Severe Hot Flashes in Women Going Through Menopause - 2 | Phase 3 Interventional | Astellas Pharma Inc | The SKYLIGHT 2 study. | 4,10 |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |