GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
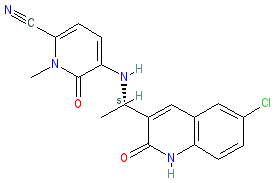
Synonyms: example 26 [US9834539B2] | FT-2102 | FT2102 | Rezlidhia®
olutasidenib is an approved drug (FDA (2022))
Compound class:
Synthetic organic
Comment: Olutasidenib (FT-2102) is an orally available and brain penetrant inhibitor of mutant IDH1 that is being developed by Forma Therapeutics for antineoplastic potential [1]. Activating mutations in IDH1 (e.g. IDH1R132H) drive cancer initiation and growth by enhancing the formation of the oncometabolite 2-hydroxyglutarate (2HG) [4]. Pharmacological inhibition of mutant IDH1 blocks 2HG production as a means to restore normal cellular differentiation. This is the same mechanism of action as ivosidenib (Tibsovo®) that was FDA approved in 2018.
FT-2102 is claimed as example 26 (I-14) in Forma Tm2's patent US9834539B2 [3]. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| FT-2102 was evaluated in clinical trials as a monotherapy or in conjunction with other chemotherapeutics (e.g. azacitidine or cytarabine), in patients with solid or liquid tumours that carry IDH1 mutations. It has been estimated that IDH1 mutations are present in 6-8% of patients with AML, and in approximately 80% of patients with grade 2 and 3 gliomas and secondary glioblastoma. Click here to link to ClinicalTrials.gov's full list of FT-2102 trials. First FDA approval was granted in December 2022 [2], for the treatment of relapsed/refractory IDH1 mutation-positive acute myeloid leukemia (AML). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03684811 | A Study of FT 2102 in Participants With Advanced Solid Tumors and Gliomas With an IDH1 Mutation | Phase 1/Phase 2 Interventional | Forma Therapeutics, Inc. | ||
| NCT02719574 | Open-label Study of FT-2102 With or Without Azacitidine or Cytarabine in Patients With AML or MDS With an IDH1 Mutation | Phase 1/Phase 2 Interventional | Forma Therapeutics, Inc. | 5 | |