GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
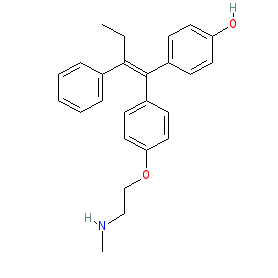
Synonyms: N-Desmethyl-4-hydroxytamoxifen | Z-Endoxifen
Compound class:
Synthetic organic
Comment: Endoxifen is the most active metabolite of tamoxifen. Endoxifen generation in vivo is performed by the cytochrome P450 (CYP) 2D6 enzyme [1,6-7,9]. In addition to its antagonistic activity at estrogen receptors, it also acts as an aromatase inhibitor [4,8].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Helland T, Henne N, Bifulco E, Naume B, Borgen E, Kristensen VN, Kvaløy JT, Lash TL, Alnæs GIG, van Schaik RH et al.. (2017)
Serum concentrations of active tamoxifen metabolites predict long-term survival in adjuvantly treated breast cancer patients. Breast Cancer Res, 19 (1): 125. [PMID:29183390] |
|
2. Lu WJ, Desta Z, Flockhart DA. (2012)
Tamoxifen metabolites as active inhibitors of aromatase in the treatment of breast cancer. Breast Cancer Res Treat, 131 (2): 473-81. [PMID:21390495] |
|
3. Lu WJ, Xu C, Pei Z, Mayhoub AS, Cushman M, Flockhart DA. (2012)
The tamoxifen metabolite norendoxifen is a potent and selective inhibitor of aromatase (CYP19) and a potential lead compound for novel therapeutic agents. Breast Cancer Res Treat, 133 (1): 99-109. [PMID:21814747] |
|
4. Lv W, Liu J, Lu D, Flockhart DA, Cushman M. (2013)
Synthesis of mixed (E,Z)-, (E)-, and (Z)-norendoxifen with dual aromatase inhibitory and estrogen receptor modulatory activities. J Med Chem, 56 (11): 4611-8. [PMID:23731360] |
|
5. Ma J, Chu Z, Lu JBL, Liu J, Zhang Q, Liu Z, Tang D. (2018)
The Cytochrome P450 Enzyme Responsible for the Production of (Z)-Norendoxifen in vitro. Chem Biodivers, 15 (1). [PMID:28834279] |
|
6. Maksina AG, Daĭniak BA, Rakhmanova IV. (1988)
[Use of the spin label method for studying the structure of the brain membranes in cats with streptomycin poisoning]. Nauchnye Doki Vyss Shkoly Biol Nauki, (11): 61-4. [PMID:2852035] |
|
7. Sanchez-Spitman A, Dezentjé V, Swen J, Moes DJAR, Böhringer S, Batman E, van Druten E, Smorenburg C, van Bochove A, Zeillemaker A et al.. (2019)
Tamoxifen Pharmacogenetics and Metabolism: Results From the Prospective CYPTAM Study. J Clin Oncol, 37 (8): 636-646. [PMID:30676859] |
|
8. Shagufta, Ahmad I. (2018)
Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur J Med Chem, 143: 515-531. [PMID:29207335] |
|
9. Skaar TC, Desta Z. (2018)
CYP2D6 and Endoxifen in Tamoxifen Therapy: A Tribute to David A. Flockhart. Clin Pharmacol Ther, 103 (5): 755-757. [PMID:29473149] |