GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
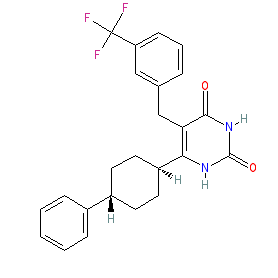
Synonyms: C-118335 | C118335 | compound 7 [PMID: 23131342] | CORT118335
Compound class:
Synthetic organic
Comment: Miricorilant (CORT118335) is a potent, orally active non-steroidal glucocorticoid receptor (GR) antagonist that is being developed by Corcept Therapeutics [2]. Selective GR antagonists are being investigated for their potential to mitigate antipsychotic-induced weight gain, a beneficial activity that was first identified using the dual GR/progesterone receptor antagonist mifepristone [1].
Hunt et al. (2010) specify CORT118335 with the InChIKey GVVUZBSCYAVFTI-IYARVYRRSA-N and as the trans isomer (or the (1r,4r) isomer) [2], which corresponds to the chemical structure submitted to the WHO for the INN miricorilant. Note that PubChem lists this compound in the form without any specified stereochemistry, with PubChem CID 66550324, and that ChEMBL record the cis (or (1s,4s)) isomer as CHEMBL2204043 (InChIKey GVVUZBSCYAVFTI-HDICACEKNA-N). Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| In a reporter gene assay (measured as inhibition of dexamethasone induced luciferase expression through the glucocorticoid response element) CORT118335 has a Ki of 24 nM [2]. CORT118335 exhibited good selectivity against PR, ER and AR, with no significant affinity in receptor binding assays, and in reporter gene assays in comparison to GR only selectivity against MR (8-fold) was noteworthy. CORT118335 did not exhibit any significant liability in terms of inhibition of any of the five major human cytochrome p450 enzymes (3A4, 2D6, 1A2, 2C9, 2C19). Preclinical studies show that CORT118335 is efficacious in the rat olanzapine-induced weight gain model. |
| Selectivity at nuclear hormone receptors | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||