GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
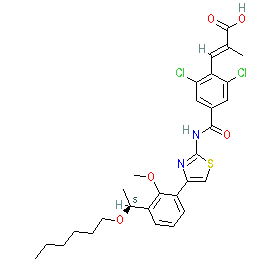
Synonyms: Mulpleta® | S-888711 | S888711
lusutrombopag is an approved drug (Japan (2015), FDA (2018), EMA (2019))
Compound class:
Synthetic organic
Comment: Lusutrombopag is an orally bioavailable, nonpeptidyl, small molecule thrombopoietin (TPO) receptor agonist [4] that was developed by Shionogi in Japan. It was the second TPO receptor agonist to receive FDA approval in 2018 (avatrombopag was the first).
Lusutrombopag demonstrates antibacterial activity in vitro with potential to be repurposed as a treatment for infections caused by drug-resistant Enterococcus spp. [3]. |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. FDA.
Lusutrombopag; FDA Approved Drug Products. Accessed on 01/08/2018. Modified on 01/08/2018. Drugs@FDA, https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process |
|
2. Kim ES. (2016)
Lusutrombopag: First Global Approval. Drugs, 76 (1): 155-8. [PMID:26666417] |
|
3. She P, Li L, Yang Y, Zhou L, Huang G, Xiao D, Wu Y. (2024)
Lusutrombopag as a Repurposing Drug in Combination with Aminoglycosides against Vancomycin-Resistant Enterococcus. ACS Infect Dis, 10 (4): 1327-1338. [PMID:38567846] |
|
4. Yoshida H, Yamada H, Nogami W, Dohi K, Kurino-Yamada T, Sugiyama K, Takahashi K, Gahara Y, Kitaura M, Hasegawa M et al.. (2018)
Development of a new knock-in mouse model and evaluation of pharmacological activities of lusutrombopag, a novel, nonpeptidyl small-molecule agonist of the human thrombopoietin receptor c-Mpl. Exp Hematol, 59: 30-39.e2. [PMID:29274361] |