GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
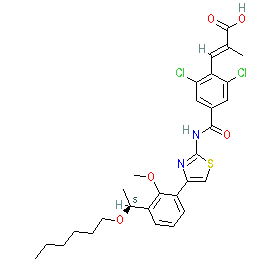
Synonyms: Mulpleta® | S-888711 | S888711
lusutrombopag is an approved drug (Japan (2015), FDA (2018), EMA (2019))
Compound class:
Synthetic organic
Comment: Lusutrombopag is an orally bioavailable, nonpeptidyl, small molecule thrombopoietin (TPO) receptor agonist [4] that was developed by Shionogi in Japan. It was the second TPO receptor agonist to receive FDA approval in 2018 (avatrombopag was the first).
Lusutrombopag demonstrates antibacterial activity in vitro with potential to be repurposed as a treatment for infections caused by drug-resistant Enterococcus spp. [3]. |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Lusutrombopag was first approved in Japan in 2015, to increase platelet count (and thus reduce bleeding risk) in patients with chronic liver disease-induced thrombocytopenia and who are scheduled for invasive surgery [2]. FDA approval for the same use was granted in July 2018 [1]. Lusutrombopag has completed Phase 2 clinical evaluation in patients with autoimmune thrombocytopenia (NCT01129024). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT01129024 | An Open-label Safety Study of S-888711 | Phase 2 Interventional | Shionogi Inc. | ||