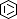GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Farnesoid X receptor
Target id: 603
Nomenclature: Farnesoid X receptor
Systematic Nomenclature: NR1H4
Contents:
- Gene and Protein Information
- Previous and Unofficial Names
- Database Links
- Selected 3D Structures
- Natural/Endogenous Ligands
- Rank order lists
- Agonists
- Antagonists
- Immunopharmacology Comments
- DNA Binding
- Main Co-regulators
- Main Target Genes
- Tissue Distribution
- Physiological Consequences of Altering Gene Expression
- Phenotypes, Alleles and Disease Models
- Clinically-Relevant Mutations and Pathophysiology
- References
- Contributors
- How to cite this page
Gene and Protein Information  |
|||||
| Species | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 486 | 12q23.1 | NR1H4 | nuclear receptor subfamily 1 group H member 4 | 11 |
| Mouse | 484 | 10 44.98 cM | Nr1h4 | nuclear receptor subfamily 1, group H, member 4 | 44 |
| Rat | 469 | 7q13 | Nr1h4 | nuclear receptor subfamily 1, group H, member 4 | 11 |
Database Links  |
|
| Alphafold | Q96RI1 (Hs), Q3V1T8 (Mm), Q62735 (Rn) |
| CATH/Gene3D | 3.30.50.10 |
| ChEMBL Target | CHEMBL2047 (Hs), CHEMBL4105917 (Rn) |
| Ensembl Gene | ENSG00000012504 (Hs), ENSMUSG00000047638 (Mm), ENSRNOG00000007197 (Rn) |
| Entrez Gene | 9971 (Hs), 20186 (Mm), 60351 (Rn) |
| Human Protein Atlas | ENSG00000012504 (Hs) |
| KEGG Gene | hsa:9971 (Hs), mmu:20186 (Mm), rno:60351 (Rn) |
| OMIM | 603826 (Hs) |
| Orphanet | ORPHA304804 (Hs) |
| Pharos | Q96RI1 (Hs) |
| RefSeq Nucleotide | NM_005123 (Hs), NM_009108 (Mm), NM_001163504 (Mm), NM_001163700 (Mm), NM_021745 (Rn) |
| RefSeq Protein | NP_005114 (Hs), NP_001157172 (Mm), NP_001156976 (Mm), NP_033134 (Mm), NP_068513 (Rn) |
| SynPHARM |
6410 (in complex with chenodeoxycholic acid) 6415 (in complex with fexaramine) 6414 (in complex with GW4064) |
| UniProtKB | Q96RI1 (Hs), Q3V1T8 (Mm), Q62735 (Rn) |
| Wikipedia | NR1H4 (Hs) |
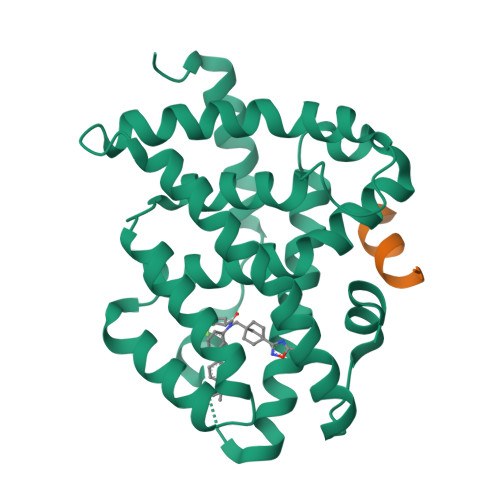
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
|
|
||||||||||||
Natural/Endogenous Ligands  |
| chenodeoxycholic acid |
| cholic acid |
| deoxycholic acid |
| lithocholic acid |
| 22R-hydroxycholesterol |
| Comments: A series of bile acids are natural ligands |
| Potency order (Human) |
| chenodeoxycholic acid > lithocholic acid, deoxycholic acid [30,35] |
Download all structure-activity data for this target as a CSV file 
Agonists  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific agonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Antagonists  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific antagonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
FXR is predominantly expressed in liver, intestine, kidney and adipose tissue, but has also been detected in immune cells (CD4+, CD8+, CD19+ and CD14+ cells) [43], albeit at much lower levels than in the primary sites of expression.FXR in inflammation:FXR protects against innate intestinal and hepatic inflammation in animal models.FXR expression is reduced in biopsy specimens from patients with the autoimmine conditions systemic lupus erythematosus (SLE) and inflammatory bowel disease (IBD). FXR activation in human monocytes (from healthy controls and MS patients) induces an anti-inflammatory phenotype facilitating control of effector T cell proliferation [16], suggesting that FXR has a regulatory effect in the control of T cell-mediated autoimmunity. This effect is IL-10 dependent. |
DNA Binding  |
|||||||
|
|||||||
| DNA Binding Comments | |||||||
| FXR binds IR1 | |||||||
Main Co-regulators  |
||||||
| Name | Activity | Specific | Ligand dependent | AF-2 dependent | Comments | References |
| PPARGC1A | Co-activator | No | Yes | Yes | 20,41,53 | |
| NCOA1 | Co-activator | No | Yes | Yes | 30,35 | |
| MED1 | Co-activator | No | Yes | Yes | 37 | |
| CARM1 | Co-activator | No | Yes | Yes | 1 | |
| PRMT1 | Co-activator | No | Yes | No | 40 | |
| TRRAP | Co-activator | No | Yes | No | 47 | |
Main Target Genes  |
|||||
| Name | Species | Effect | Technique | Comments | References |
| VLDLR | Human | Activated | 6 | ||
| NR0B2 | Human | Activated | Transient transfection, EMSA | NR0B2 is a nuclear receptor also commonly known as short heterodimer partner (SHP) | 13,27 |
| ABCB11 | Human | Activated | Transient transfection, EMSA | progressive familial intrahepatic cholestasis 2, bile salt export pump | 1 |
| FABP6 | Human | Activated | Transient transfection, EMSA | Fatty acid binding protein 6, ileal | 14 |
| ABCB4 | Human | Activated | Transient transfection, EMSA | 7 | |
| FGF19 | Human | Activated | Transient transfection, EMSA | 15 | |
| ABCC2 | Human | Activated | Transient transfection, EMSA | multidrug resistance associated protein 2 (MRP2multidrug resistance associated protein 2 (MRP2) | 29 |
| SLCO1B3 | Human | Activated | Transient transfection, EMSA | solute carrier organic anion transporter family member 1B3 (OATP1B3; SLCO1B3) | 19 |
| SLC27A5 | Human | Activated | Transient transfection, EMSA | bile acid-CoA synthetase (BACS) | 38 |
| SLC3A1 | Human | Activated | Transient transfection, EMSA | bile acid-CoA: amino acid N-acetyltransferase (BAT) | 38 |
| APOAI | Human | Repressed | Transient transfection, EMSA | apolipoprotein A-I (APOAI): Repressed by FXR binding as a monomer to negative FXR response element. | 6 |
| APOC2 | Human | Repressed | Transient transfection, EMSA | apolipoprotein C-II (ApoCII) | 21 |
| APOE | Human | Activated | APOE- apolipoprotein E. | 29 | |
| C3 | Human | Activated | Transient transfection, EMSA | complement component 3 | 26 |
| PDK4 | Human | Activated | pyruvate dehydrogenase kinase, isozyme 4 | 41 | |
| PLTP | Human | Activated | Transient transfection, EMSA | phospholipid transfer protein | 48 |
| PPARA | Human | Activated | Transient transfection, EMSA | The mouse PPARa gene is not induced. | 37 |
| Fibrinogen | Human | Activated | 2 | ||
| KNG1 | Human | Activated | Transient transfection, EMSA | 7 | |
| SDC1 | Human | Activated | Transient transfection, EMSA | CD138, syndecan-1, SYND1 | 9 |
| VIPR1 | Human | Activated | 5 | ||
| alpha-crystallin | Human | Activated | Transient transfection, EMSA | 25 | |
| organic solute transporter α-β | Human | Activated | 12,24,55 | ||
| Abcb4 | Mouse | Activated | Transient transfection, EMSA | 17-18 | |
| Apoc3 | Mouse | Repressed | ChIP, Transient transfection, EMSA | 6 | |
Tissue Distribution 
|
||||||||||
|
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||
|
||||||||
References
1. Ananthanarayanan M, Li S, Balasubramaniyan N, Suchy FJ, Walsh MJ. (2004) Ligand-dependent activation of the farnesoid X-receptor directs arginine methylation of histone H3 by CARM1. J Biol Chem, 279 (52): 54348-57. [PMID:15471871]
2. Anisfeld AM, Kast-Woelbern HR, Lee H, Zhang Y, Lee FY, Edwards PA. (2005) Activation of the nuclear receptor FXR induces fibrinogen expression: a new role for bile acid signaling. J Lipid Res, 46 (3): 458-68. [PMID:15604525]
3. Carpenter J, Wu G, Wang Y, Cook EM, Wang T, Sitkoff D, Rossi KA, Mosure K, Zhuo X, Cao GG et al.. (2021) Discovery of BMS-986318, a Potent Nonbile Acid FXR Agonist for the Treatment of Nonalcoholic Steatohepatitis. ACS Med Chem Lett, 12 (9): 1413-1420. [PMID:34531950]
4. Chianelli D, Rucker PV, Roland J, Tully DC, Nelson J, Liu X, Bursulaya B, Hernandez ED, Wu J, Prashad M et al.. (2020) Nidufexor (LMB763), a Novel FXR Modulator for the Treatment of Nonalcoholic Steatohepatitis. J Med Chem, 63 (8): 3868-3880. [PMID:31940200]
5. Chignard N, Mergey M, Barbu V, Finzi L, Tiret E, Paul A, Housset C. (2005) VPAC1 expression is regulated by FXR agonists in the human gallbladder epithelium. Hepatology, 42 (3): 549-57. [PMID:16037943]
6. Claudel T, Sturm E, Duez H, Torra IP, Sirvent A, Kosykh V, Fruchart JC, Dallongeville J, Hum DW, Kuipers F, Staels B. (2002) Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J Clin Invest, 109 (7): 961-71. [PMID:11927623]
7. Cui J, Huang L, Zhao A, Lew JL, Yu J, Sahoo S, Meinke PT, Royo I, Pelaez F, Wright SD. (2003) Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J Biol Chem, 278 (12): 10214-20. [PMID:12525500]
8. Deng R, Yang D, Yang J, Yan B. (2006) Oxysterol 22(R)-hydroxycholesterol induces the expression of the bile salt export pump through nuclear receptor farsenoid X receptor but not liver X receptor. J Pharmacol Exp Ther, 317 (1): 317-25. [PMID:16371446]
9. Downes M, Verdecia MA, Roecker AJ, Hughes R, Hogenesch JB, Kast-Woelbern HR, Bowman ME, Ferrer JL, Anisfeld AM, Edwards PA, Rosenfeld JM, Alvarez JG, Noel JP, Nicolaou KC, Evans RM. (2003) A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol Cell, 11 (4): 1079-92. [PMID:12718892]
10. Festa C, Finamore C, Marchianò S, Di Leva FS, Carino A, Monti MC, Del Gaudio F, Ceccacci S, Limongelli V, Zampella A et al.. (2019) Investigation around the Oxadiazole Core in the Discovery of a New Chemotype of Potent and Selective FXR Antagonists. ACS Med Chem Lett, 10 (4): 504-510. DOI: 10.1021/acsmedchemlett.8b00534 [PMID:30996787]
11. Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. (1995) Identification of a nuclear receptor that is activated by farnesol metabolites. Cell, 81 (5): 687-93. [PMID:7774010]
12. Frankenberg T, Rao A, Chen F, Haywood J, Shneider BL, Dawson PA. (2006) Regulation of the mouse organic solute transporter alpha-beta, Ostalpha-Ostbeta, by bile acids. Am J Physiol Gastrointest Liver Physiol, 290 (5): G912-22. [PMID:16357058]
13. Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. (2000) A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell, 6 (3): 517-26. [PMID:11030332]
14. Grober J, Zaghini I, Fujii H, Jones SA, Kliewer SA, Willson TM, Ono T, Besnard P. (1999) Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J Biol Chem, 274 (42): 29749-54. [PMID:10514450]
15. Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. (2003) Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev, 17 (13): 1581-91. [PMID:12815072]
16. Hucke S, Herold M, Liebmann M, Freise N, Lindner M, Fleck AK, Zenker S, Thiebes S, Fernandez-Orth J, Buck D et al.. (2016) The farnesoid-X-receptor in myeloid cells controls CNS autoimmunity in an IL-10-dependent fashion. Acta Neuropathol, 132 (3): 413-31. [PMID:27383204]
17. Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. (2005) Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab, 2 (4): 217-25. [PMID:16213224]
18. Jaye MC, Krawiec JA, Campobasso N, Smallwood A, Qiu C, Lu Q, Kerrigan JJ, De Los Frailes Alvaro M, Laffitte B, Liu WS, Marino JP, Meyer CR, Nichols JA, Parks DJ, Perez P, Sarov-Blat L, Seepersaud SD, Steplewski KM, Thompson SK, Wang P, Watson MA, Webb CL, Haigh D, Caravella JA, Macphee CH, Willson TM, Collins JL. (2005) Discovery of substituted maleimides as liver X receptor agonists and determination of a ligand-bound crystal structure. J Med Chem, 48 (17): 5419-22. [PMID:16107141]
19. Jung D, Podvinec M, Meyer UA, Mangelsdorf DJ, Fried M, Meier PJ, Kullak-Ublick GA. (2002) Human organic anion transporting polypeptide 8 promoter is transactivated by the farnesoid X receptor/bile acid receptor. Gastroenterology, 122 (7): 1954-66. [PMID:12055601]
20. Kanaya E, Shiraki T, Jingami H. (2004) The nuclear bile acid receptor FXR is activated by PGC-1alpha in a ligand-dependent manner. Biochem J, 382 (Pt 3): 913-21. [PMID:15202934]
21. Kast HR, Nguyen CM, Sinal CJ, Jones SA, Laffitte BA, Reue K, Gonzalez FJ, Willson TM, Edwards PA. (2001) Farnesoid X-activated receptor induces apolipoprotein C-II transcription: a molecular mechanism linking plasma triglyceride levels to bile acids. Mol Endocrinol, 15 (10): 1720-8. [PMID:11579204]
22. Kinzel O, Steeneck C, Kremoser C. (2019) FXR (NR1H4) binding and activity modulating compounds. Patent number: US10485795B2. Assignee: Gilead Sciences Inc. Priority date: 13/07/2011. Publication date: 26/11/2019.
23. Kok T, Hulzebos CV, Wolters H, Havinga R, Agellon LB, Stellaard F, Shan B, Schwarz M, Kuipers F. (2003) Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J Biol Chem, 278 (43): 41930-7. [PMID:12917447]
24. Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. (2006) The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am J Physiol Gastrointest Liver Physiol, 290 (3): G476-85. [PMID:16269519]
25. Lee FY, Kast-Woelbern HR, Chang J, Luo G, Jones SA, Fishbein MC, Edwards PA. (2005) Alpha-crystallin is a target gene of the farnesoid X-activated receptor in human livers. J Biol Chem, 280 (36): 31792-800. [PMID:16012168]
26. Li J, Pircher PC, Schulman IG, Westin SK. (2005) Regulation of complement C3 expression by the bile acid receptor FXR. J Biol Chem, 280 (9): 7427-34. [PMID:15590640]
27. Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. (2000) Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell, 6 (3): 507-15. [PMID:11030331]
28. Maisonneuve IM, Archer S, Glick SD. (1994) U50,488, a kappa opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett, 181: 57-60. [PMID:7898771]
29. Mak PA, Kast-Woelbern HR, Anisfeld AM, Edwards PA. (2002) Identification of PLTP as an LXR target gene and apoE as an FXR target gene reveals overlapping targets for the two nuclear receptors. J Lipid Res, 43 (12): 2037-41. [PMID:12454263]
30. Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. (1999) Identification of a nuclear receptor for bile acids. Science, 284 (5418): 1362-5. [PMID:10334992]
31. Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD, Creech KL, Moore LB, Wilson JG, Lewis MC, Jones SA, Willson TM. (2000) Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem, 43 (16): 2971-4. [PMID:10956205]
32. Mi LZ, Devarakonda S, Harp JM, Han Q, Pellicciari R, Willson TM, Khorasanizadeh S, Rastinejad F. (2003) Structural basis for bile acid binding and activation of the nuclear receptor FXR. Mol Cell, 11 (4): 1093-100. [PMID:12718893]
33. Mo C, Xu X, Zhang P, Peng Y, Zhao X, Chen S, Guo F, Xiong Y, Chu XJ, Xu X. (2023) Discovery of HPG1860, a Structurally Novel Nonbile Acid FXR Agonist Currently in Clinical Development for the Treatment of Nonalcoholic Steatohepatitis. J Med Chem, 66 (14): 9363-9375. [PMID:37424079]
34. Nara SJ, Jogi S, Cheruku S, Kandhasamy S, Jaipuri F, Kathi PK, Reddy S, Sarodaya S, Cook EM, Wang T et al.. (2022) Discovery of BMS-986339, a Pharmacologically Differentiated Farnesoid X Receptor Agonist for the Treatment of Nonalcoholic Steatohepatitis. J Med Chem, 65 (13): 8948-8960. [PMID:35704802]
35. Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science, 284 (5418): 1365-8. [PMID:10334993]
36. Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM. (2002) 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem, 45 (17): 3569-72. [PMID:12166927]
37. Pineda Torra I, Freedman LP, Garabedian MJ. (2004) Identification of DRIP205 as a coactivator for the Farnesoid X receptor. J Biol Chem, 279 (35): 36184-91. [PMID:15187081]
38. Pircher PC, Kitto JL, Petrowski ML, Tangirala RK, Bischoff ED, Schulman IG, Westin SK. (2003) Farnesoid X receptor regulates bile acid-amino acid conjugation. J Biol Chem, 278 (30): 27703-11. [PMID:12754200]
39. Rizzo G, Passeri D, De Franco F, Ciaccioli G, Donadio L, Rizzo G, Orlandi S, Sadeghpour B, Wang XX, Jiang T et al.. (2010) Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol, 78 (4): 617-30. [PMID:20631053]
40. Rizzo G, Renga B, Antonelli E, Passeri D, Pellicciari R, Fiorucci S. (2005) The methyl transferase PRMT1 functions as co-activator of farnesoid X receptor (FXR)/9-cis retinoid X receptor and regulates transcription of FXR responsive genes. Mol Pharmacol, 68 (2): 551-8. [PMID:15911693]
41. Savkur RS, Thomas JS, Bramlett KS, Gao Y, Michael LF, Burris TP. (2005) Ligand-dependent coactivation of the human bile acid receptor FXR by the peroxisome proliferator-activated receptor gamma coactivator-1alpha. J Pharmacol Exp Ther, 312 (1): 170-8. [PMID:15329387]
42. Schierle S, Neumann S, Heitel P, Willems S, Kaiser A, Pollinger J, Merk D. (2020) Design and Structural Optimization of Dual FXR/PPARδ Activators. J Med Chem, 63 (15): 8369-8379. [PMID:32687365]
43. Schote AB, Turner JD, Schiltz J, Muller CP. (2007) Nuclear receptors in human immune cells: expression and correlations. Mol Immunol, 44 (6): 1436-45. [PMID:16837048]
44. Seol W, Choi HS, Moore DD. (1995) Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol, 9 (1): 72-85. [PMID:7760852]
45. Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. (2000) Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell, 102 (6): 731-44. [PMID:11030617]
46. Tully DC, Rucker PV, Chianelli D, Williams J, Vidal A, Alper PB, Mutnick D, Bursulaya B, Schmeits J, Wu X et al.. (2017) Discovery of Tropifexor (LJN452), a Highly Potent Non-bile Acid FXR Agonist for the Treatment of Cholestatic Liver Diseases and Nonalcoholic Steatohepatitis (NASH). J Med Chem, 60 (24): 9960-9973. [PMID:29148806]
47. Unno A, Takada I, Takezawa S, Oishi H, Baba A, Shimizu T, Tokita A, Yanagisawa J, Kato S. (2005) TRRAP as a hepatic coactivator of LXR and FXR function. Biochem Biophys Res Commun, 327 (3): 933-8. [PMID:15649435]
48. Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, Gonzalez FJ, Heyman RA, Mangelsdorf DJ, Moore DD. (2002) A natural product that lowers cholesterol as an antagonist ligand for FXR. Science, 296 (5573): 1703-6. [PMID:11988537]
49. Wang H, Chen J, Hollister K, Sowers LC, Forman BM. (1999) Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell, 3 (5): 543-53. [PMID:10360171]
50. Wu J, Xia C, Meier J, Li S, Hu X, Lala DS. (2002) The hypolipidemic natural product guggulsterone acts as an antagonist of the bile acid receptor. Mol Endocrinol, 16 (7): 1590-7. [PMID:12089353]
51. Yan Y, Wang W, Yan A, Zhu H, Meng Q. (2025) β-sitosterol protects against ANIT-induced hepatotoxicity and cholestasis via FXR activation. Toxicol In Vitro, 104: 106020. [PMID:39947414]
52. Yu DD, Lin W, Forman BM, Chen T. (2014) Identification of trisubstituted-pyrazol carboxamide analogs as novel and potent antagonists of farnesoid X receptor. Bioorg Med Chem, 22 (11): 2919-38. [PMID:24775917]
53. Zhang Y, Castellani LW, Sinal CJ, Gonzalez FJ, Edwards PA. (2004) Peroxisome proliferator-activated receptor-gamma coactivator 1alpha (PGC-1alpha) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev, 18 (2): 157-69. [PMID:14729567]
54. Zhang Y, Kast-Woelbern HR, Edwards PA. (2003) Natural structural variants of the nuclear receptor farnesoid X receptor affect transcriptional activation. J Biol Chem, 278 (1): 104-10. [PMID:12393883]
55. Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, Trauner M. (2006) Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol, 290 (5): G923-32. [PMID:16357057]