GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: NN-9535 | NN9535 | NNC0480 0389 | Ozempic® (injectable semaglutide) | Rybelsus® (oral semaglutide) | Wegovy®
semaglutide is an approved drug (FDA (2017), EMA (2018))
Compound class:
Peptide
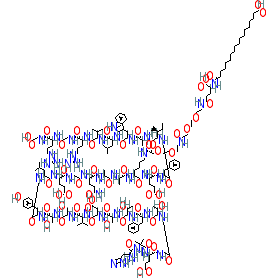
Comment: Semaglutide is a glucagon like peptide 1 (GLP-1) receptor peptidomimetic agonist [9]. Structurally it is a modified analogue of glucagon-like peptide 1-(7-37) with amino acids at positions 8 and 34 replaced by α-aminobutyric acid and arginine respectively, and Lys26 is acylated with stearic diacid. Semaglutide has slightly lower GLP-1 receptor affinity than liraglutide, but this is compensated by an increased serum albumin affinity, which ensures improved resistance to metabolic degradation.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||
| References |
|
1. AbuAlrob MA, Itbaisha A, Abujwaid YK, Abulehia A, Hussein A, Mesraoua B. (2025)
Exploring the neuroprotective role of GLP-1 agonists against Alzheimer's disease: Real-world evidence from a propensity-matched cohort. J Alzheimers Dis Rep, 9: 25424823251388650. [PMID:41122341] |
|
2. Alkhatib M, Almasri N, Alshwayyat S, Almahariq H, Hammadeh BM, Taimeh Z, Alkhatib L, Alshwayat A, A Saadeh N, Al-Kurdi MA. (2025)
The multifaceted effects of semaglutide: exploring its broad therapeutic applications. Future Sci OA, 11 (1): 2483607. [PMID:40904035] |
|
3. Capone F, Nambiar N, Schiattarella GG. (2024)
Beyond Weight Loss: the Emerging Role of Incretin-Based Treatments in Cardiometabolic HFpEF. Curr Opin Cardiol, 39 (3): 148-153. [PMID:38294187] |
|
4. Cimino G, Vaduganathan M, Lombardi CM, Pagnesi M, Vizzardi E, Tomasoni D, Adamo M, Metra M, Inciardi RM. (2024)
Obesity, heart failure with preserved ejection fraction, and the role of glucagon-like peptide-1 receptor agonists. ESC Heart Fail, 11 (2): 649-661. [PMID:38093506] |
|
5. Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. (2017)
Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial. JAMA, 318 (15): 1460-1470. [PMID:29049653] |
|
6. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD et al.. (2019)
Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med, 381 (9): 841-851. [PMID:31185157] |
|
7. Kobak KA, Chiao YA. (2023)
Semaglutide in Heart Failure With Preserved Ejection Fraction: Benefits Above and Beyond Weight Loss. JACC Basic Transl Sci, 8 (10): 1315-1317. [PMID:38094694] |
|
8. Kosiborod MN, Petrie MC, Borlaug BA, Butler J, Davies MJ, Hovingh GK, Kitzman DW, Møller DV, Treppendahl MB, Verma S et al.. (2024)
Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes. N Engl J Med, 390 (15): 1394-1407. [PMID:38587233] |
|
9. Lau J, Bloch P, Schäffer L, Pettersson I, Spetzler J, Kofoed J, Madsen K, Knudsen LB, McGuire J, Steensgaard DB et al.. (2015)
Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J Med Chem, 58 (18): 7370-80. [PMID:26308095] |
|
10. Novo Nordisk Global: Press releases.
Novo Nordisk A/S: Evoke phase 3 trials did not demonstrate a statistically significant reduction in Alzheimer's disease progression. Accessed on 25/11/2025. Modified on 25/11/2025. novonordisk.com, https://www.novonordisk.com/news-and-media/news-and-ir-materials/news-details.html?id=916462 |
|
11. Sato R, von Haehling S. (2024)
One small STEP, one giant leap: Semaglutide in the obese phenotype of heart failure with preserved ejection fraction. Med, 5 (3): 181-183. [PMID:38460497] |
|
12. Stefano GB, Büttiker P, Weissenberger S, Raboch J, Anders M. (2025)
Semaglutide and the pathogenesis of progressive neurodegenerative disease: the central role of mitochondria. Front Neuroendocrinol, 79: 101217. [PMID:41047006] |
|
13. Zheng M, Zhang Q, Siebert HC, Loers G, Wen M, Wang Q, Zhang R, Han J, Zhang N. (2026)
Semaglutide attenuates Alzheimer's disease model progression by targeting microglial NLRP3 inflammasome-mediated neuroinflammation and ferroptosis. Exp Neurol, 396: 115537. [PMID:41173224] |