GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
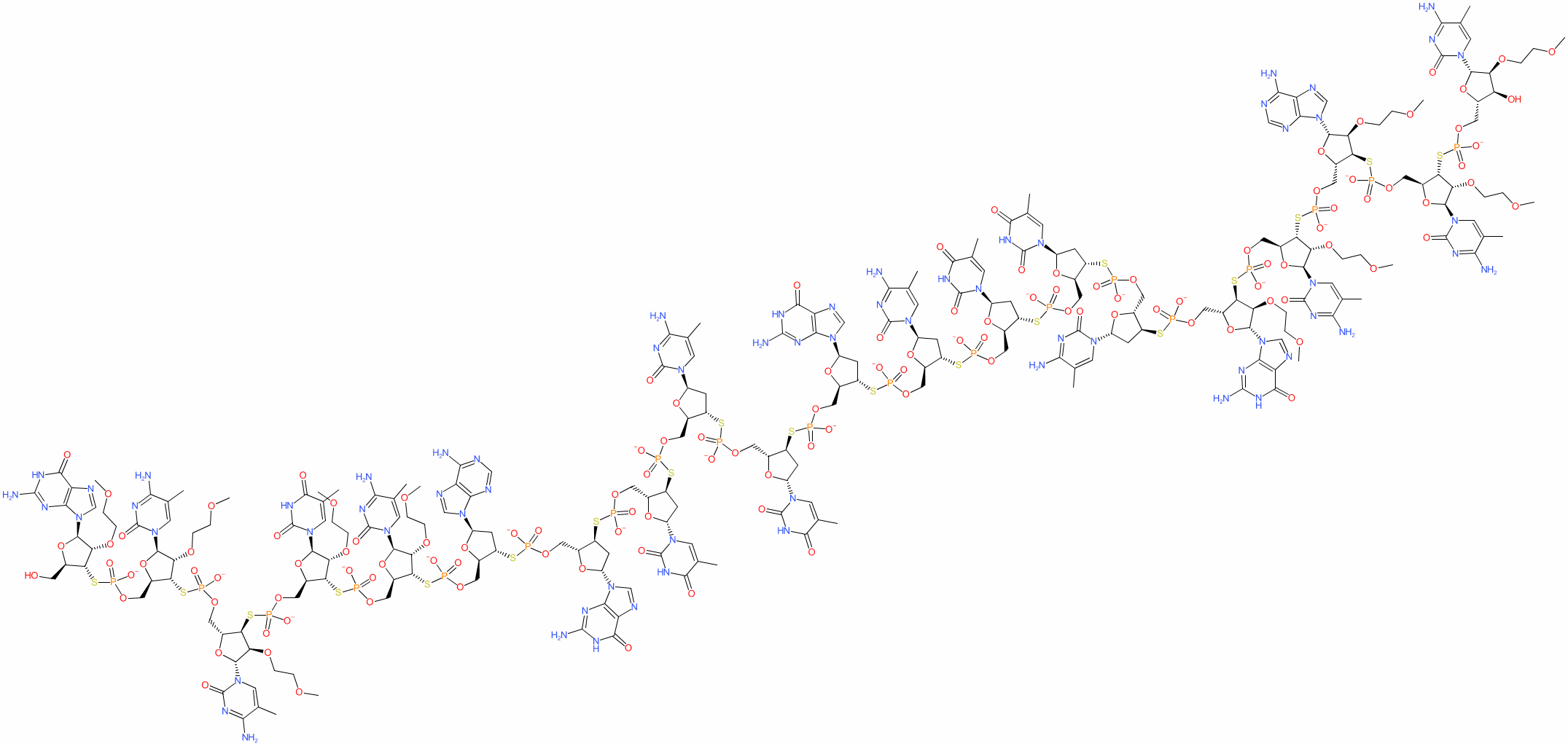
Synonyms: ISIS 301012 parent acid | isis-301012 | Kynamro®
mipomersen is an approved drug (FDA (2013))
Compound class:
Nucleic acid
Comment: Mipomersen is a synthetic phosphorothioate antisense oligonucleotide (ASO) that is designed to block hepatic production of apolipoprotein B-100 as a mechanism to reduce levels of very low-density lipoprotein (vLDL) and LDL. It is a second generation 2′-O-methoxyethyl containing ASO that is formulated as a sodium adduct for clinical administration.
|
|
|||||||||||||||
| Bioactivity Comments |
| We do not list RNA as a drug target, although the specificity of this compound is unquestionable, there is no classical affinity data for the interaction between the drug and it's mRNA target. Therefore we have not tagged a primary drug target. |