GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Abbreviated name: CBD
Synonyms: Epidiolex® | GWP42003-P | nabiximols (CBD + THC, fixed-dose oral spray) | Sativex® (CBD + THC, fixed-dose oral spray)
cannabidiol is an approved drug (FDA (2018), EMA (2019), UK (2019))
Compound class:
Natural product
Comment: Cannabidiol (CBD) is a natural extract from Cannabis plants (a 'cannabinoid'), which has recently been approved as a much needed therapy for the treatment of orphan pediatric epilepsy syndromes. It may also have benefit in schizophrenia or post-traumatic stress disorder. The major advantage of CBD over other cannabinoids such as Δ9-tetrahydrocannabinol, is that it is devoid of psychotropic effects, whilst retaining analgesic, anti-inflammatory and other beneficial actions.
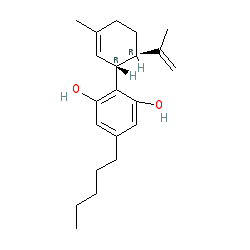
CBD is also commonly represented with the structure shown by CID 26346. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| In June 2018 the FDA approved an oral solution containing CBD as a treatment for seizures associated with Lennox-Gastaut syndrome and Dravet syndrome in paediatric patients who are ≥2 years old. Lennox-Gastaut and Dravet syndromes are rare, severe and debilitating forms of childhood epilepsy. This is the first treatment for Dravet syndrome to be approved. The Epidiolex® formulation (a liquid formulation of pure plant-derived CBD) was reported to have met its primary endpoint in Phase 3 clinical trials (see NCT02091375 and NCT02224703 for current trial details), which were designed to evaluate the ability of this cannibinoid to reduce convulsive seizures in children with Dravet syndrome. Prior to formal approval, Epidiolex® had both Orphan Drug Designation and Fast Track Designation from the US FDA for the treatment of Dravet syndrome. The EMA has granted orphan drug designation for the treatment of pediatric epilepsy syndromes (Dravet, Lennox-Gestaut, and West syndromes), GvHD, perinatal asphyxia and tuberous sclerosis. Epidiolex® is also being evaluated in tuberous sclerosis complex, another rare and severe pediatric epilepsy disorder. Click here to link to ClinicalTrials.gov's list of Phase 3 Epidiolex® trials. The manufacturer of Epidiolex® is GW Pharmaceuticals and their pipeline page provides details of the other cannabinoid drug candidates and additional conditions that they are investigating. A fixed-dose oral spray (Sativex®, nabiximols) containing CBD and THC has been approved in some countries as a prescription-only medicine for the treatment of spasticity due to multiple sclerosis. |
Mechanism Of Action and Pharmacodynamic Effects  |
| CBD is not an agonist at CB1 cannabinoid receptors. The molecular mechanisms of action of CBD are not precisely defined, but may involve multiple targets. For example, CBD has been shown to stimulate vanilloid pain receptors (TRPVs), and to modulate cellular uptake and enzymatic hydrolysis of endogenous anandamide, and these mechanisms may contribute towards the drug's pharmacological effects [1]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02091375 | Antiepileptic Efficacy Study of GWP42003-P in Children and Young Adults With Dravet Syndrome (GWPCARE1) | Phase 3 Interventional | GW Research Ltd | ||
| NCT02224703 | GWPCARE2 A Study to Investigate the Efficacy and Safety of Cannabidiol (GWP42003-P) in Children and Young Adults With Dravet Syndrome | Phase 3 Interventional | GW Research Ltd | ||
| NCT05288283 | Efficacy and Safety of GWP42003-P Oral Solution in Children With Epilepsy With Myoclonic-atonic Seizures | Phase 3 Interventional | Jazz Pharmaceuticals | ||