GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
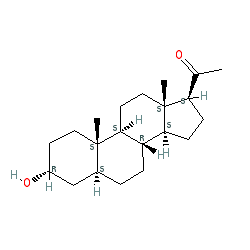
Synonyms: 3α,5α-tetrahydroprogesterone | 3α,5α-THP | allopregnanolone | SAGE-547 | SAGE547 | Zulresso®
5α-pregnan-3α-ol-20-one is an approved drug (FDA (2019))
Compound class:
Metabolite
Comment: This steroidal compound behaves as a neuroactive agent with anti-depressant activity in mood disorders [2-3]. Pharmacologically it is a positive allosteric modulator of GABAA receptors.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| As the INN brexanolone (research code SAGE-547), this drug was approved by the FDA in March 2019, indicated for the treatment of moderate to severe postpartum depression [1]. Brexanolone has to be delivered as a continuous i.v. infusion (over a period of 60 hours), and this must only be carried out by a health care provider in a certified health care facility. In the US brexanolone is only available through a restricted distribution program called the Zulresso REMS Program. |