GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Natural product
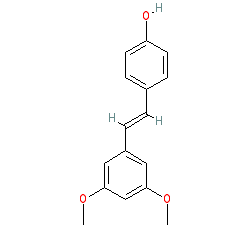
Comment: Pterostilbene is a phytomolecule present in blueberries and grapes [2].
|
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Pterostilbene is proposed to exert a neuroprotective effect (combating Aβ-induced apoptosis in neuronal cells) through a novel PI3K/Akt/Bad signalling pathway that impacts upon downstream mitochondria-mediated and caspase-dependent pathways [1]. In vivo, pterostilbene treatment induces anti-amyloidogenic and anti-inflammatory effects [3], and enhances cognitive function in APP/PS1 Alzheimer's model mice. |
| Selectivity at nuclear hormone receptors | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||