GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
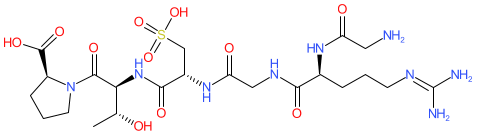
Synonyms: ALG-1001 | ALG1001 | Luminate®
Compound class:
Peptide
|
|
|||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Risuteganib (ALG-1001) is a clinical candidate for the treament of (dry) age-related macular degeneration (AMD) and diabetic macular edema (DME). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03626636 | A Clinical Trial Designed To Evaluate The Safety And Exploratory Efficacy Of 1.0 Mg Luminate® (Alg-1001) As A Treatment For Non-Exudative Macular Degeneration | Phase 2 Interventional | Allegro Ophthalmics, LLC | 2 | |
| NCT01749891 | A Safety And Efficacy Study Of ALG-1001 In Human Subjects With Wet Age-Related Macular Degeneration | Phase 1/Phase 2 Interventional | Allegro Ophthalmics, LLC | ||
| NCT02153476 | A Safety And Efficacy Study Of Alg-1001 In Human Subjects With Symptomatic Focal Vitreomacular Adhesion | Phase 2 Interventional | Allegro Ophthalmics, LLC | ||
| NCT02348918 | Phase 2 Randomized Clinical Trial of Luminate® as Compared to Avastin® in the Treatment of Diabetic Macular Edema | Phase 2 Interventional | Allegro Ophthalmics, LLC | ||