GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Natural product
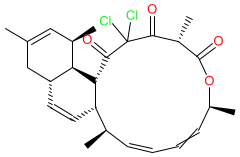
Comment: Chlorotonil A is a macrolide produced by the myxobacterium Sorangium cellulosum [2]. It has antibacterial activity against Gram-positive bacteria, antifungal activity and antimalarial activity.
The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| The MIC for antibacterial activity in vitro against a panel of Clostridioides difficile strains is in the range of 0.4-1.6 μg/ml [1]. In Plasmodium falciparum, chlorotonil A has activity against all blood stages of the parasite lifecycle (see table below) [3]. |
| Whole organism assay data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click on species/strain names for details | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||