GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
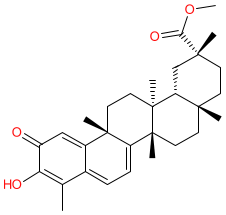
Synonyms: celastrol methyl ester
Compound class:
Natural product
Comment: Pristimerin is a naturally ocurring terpenoid compound that has been isolated from the seeds of the plant Celastrus paniculatus. It has been attributed with multiple biological activities, and is the subject of medicinal chemistry efforts to produce bioactive synthetic derivatives with therapeutic potential [2]. Molecular targets and mechanism of action are yet to be fully determined.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Chen Z, Zhang D, Yan S, Hu C, Huang Z, Li Z, Peng S, Li X, Zhu Y, Yu H et al.. (2019)
SAR study of celastrol analogs targeting Nur77-mediated inflammatory pathway. Eur J Med Chem, 177: 171-187. [PMID:31132532] |
|
2. Fu X, Jiao Y, Feng Y, Lin F, Zhang B, Mao Q, Wang J, Jiang W, Mou Y, Wang H et al.. (2024)
Scaffold Hopping of Pristimerin Provides Derivatives Containing a Privileged Quinoxaline Substructure as Potent Autophagy Inducers in Breast Cancer Cells. J Nat Prod, 87 (8): 1952-1964. [PMID:39106494] |
|
3. Ryu YB, Park SJ, Kim YM, Lee JY, Seo WD, Chang JS, Park KH, Rho MC, Lee WS. (2010)
SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorg Med Chem Lett, 20 (6): 1873-6. [PMID:20167482] |
|
4. Xiong F, Ding X, Zhang H, Luo X, Chen K, Jiang H, Luo C, Xu H. (2021)
Discovery of novel reversible monoacylglycerol lipase inhibitors via docking-based virtual screening. Bioorg Med Chem Lett, 41: 127986. [PMID:33766770] |