GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
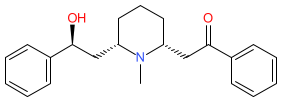
Synonyms: α-lobeline | (-)-lobeline
Compound class:
Natural product
Comment: Lobeline is a phytochemical from Lobelia species. It has been proposed to offer therapeutic benefit in a range of human disease states. At the molecular level lobeline has been reported as a nicotinic acetylcholine receptor agonist [1].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Edink E, Akdemir A, Jansen C, van Elk R, Zuiderveld O, de Kanter FJ, van Muijlwijk-Koezen JE, Smit AB, Leurs R, de Esch IJ. (2012)
Structure-based design, synthesis and structure-activity relationships of dibenzosuberyl- and benzoate-substituted tropines as ligands for acetylcholine-binding protein. Bioorg Med Chem Lett, 22 (3): 1448-54. [PMID:22243960] |
|
2. Zhao L, Ma H, Jiang Y, Li Y, Qiao L, Chen Y, Jiang X, Wang L, Wang S, Fan X. (2024)
Identification of an m6A Natural Inhibitor, Lobeline, That Reverses Lenvatinib Resistance in Hepatocellular Tumors. J Nat Prod, 87 (8): 1983-1993. [PMID:39136667] |