GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Natural product
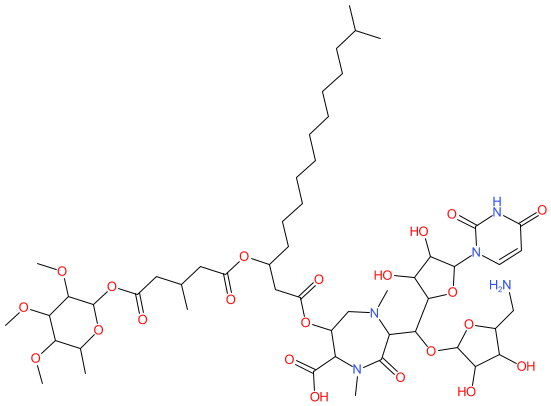
Comment: Caprazamycin B is a liponucleoside antibacterial compound, with activity against Gram-positive bacteria and mycobacteria, that was obtained from the culture broth of the actinomycete strain Streptomyces sp. MK730-62F2 [3-4]. It inhibits phospho-N-acetylmuramoyl-pentapeptide-transferase (MraY, translocase I) [5], an essential enzyme in bacterial peptidoglycan biosynthesis.
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. ANDERSON JS, MATSUHASHI M, HASKIN MA, STROMINGER JL. (1965)
LIPID-PHOSPHOACETYLMURAMYL-PENTAPEPTIDE AND LIPID-PHOSPHODISACCHARIDE-PENTAPEPTIDE: PRESUMED MEMBRANE TRANSPORT INTERMEDIATES IN CELL WALL SYNTHESIS. Proc Natl Acad Sci U S A, 53 (4): 881-9. [PMID:14324547] |
|
2. Heydanek Jr MG, Neuhaus FC. (1969)
The initial stage in peptidoglycan synthesis. IV. Solubilization of phospho-N-acetylmuramyl-pentapeptide translocase. Biochemistry, 8 (4): 1474-81. [PMID:5805290] |
|
3. Igarashi M, Nakagawa N, Doi N, Hattori S, Naganawa H, Hamada M. (2003)
Caprazamycin B, a novel anti-tuberculosis antibiotic, from Streptomyces sp. J Antibiot (Tokyo), 56 (6): 580-3. [PMID:12931868] |
|
4. Igarashi M, Takahashi Y, Shitara T, Nakamura H, Naganawa H, Miyake T, Akamatsu Y. (2005)
Caprazamycins, novel lipo-nucleoside antibiotics, from Streptomyces sp. II. Structure elucidation of caprazamycins. J Antibiot (Tokyo), 58 (5): 327-37. [PMID:16060385] |
|
5. Ishizaki Y, Hayashi C, Inoue K, Igarashi M, Takahashi Y, Pujari V, Crick DC, Brennan PJ, Nomoto A. (2013)
Inhibition of the first step in synthesis of the mycobacterial cell wall core, catalyzed by the GlcNAc-1-phosphate transferase WecA, by the novel caprazamycin derivative CPZEN-45. J Biol Chem, 288 (42): 30309-30319. [PMID:23986448] |
|
6. STRUVE WG, NEUHAUS FC. (1965)
EVIDENCE FOR AN INITIAL ACCEPTOR OF UDP-NAC-MURAMYL-PENTAPEPTIDE IN THE SYNTHESIS OF BACTERIAL MUCOPEPTIDE. Biochem Biophys Res Commun, 18: 6-12. [PMID:14265759] |