GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: albomycetin | amaromycin | picromycin
Compound class:
Natural product
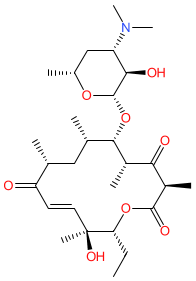
Comment: Pikromycin is a 14-membered macrolide (ketolide subclass) antibacterial compound. It was the first macrolide to be discovered, being isolated from Streptomyces venezuelae by Brockmann and Henkel in 1950 [2].
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Almutairi MM, Svetlov MS, Hansen DA, Khabibullina NF, Klepacki D, Kang HY, Sherman DH, Vázquez-Laslop N, Polikanov YS, Mankin AS. (2017)
Co-produced natural ketolides methymycin and pikromycin inhibit bacterial growth by preventing synthesis of a limited number of proteins. Nucleic Acids Res, 45 (16): 9573-9582. [PMID:28934499] |
|
2. Muxfeldt H, Shrader S, Hansen P, Brockman H. (1968)
The structure of pikromycin. J Am Chem Soc, 90 (17): 4748-9. [PMID:5661144] |