GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
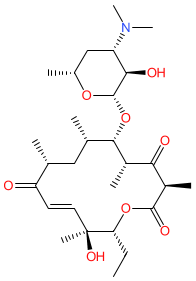
Synonyms: albomycetin | amaromycin | picromycin
Compound class:
Natural product
Comment: Pikromycin is a 14-membered macrolide (ketolide subclass) antibacterial compound. It was the first macrolide to be discovered, being isolated from Streptomyces venezuelae by Brockmann and Henkel in 1950 [2].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Pikromycin inhibits translation, leading to inhibition of bacterial growth, by binding in the nascent peptide exit tunnel (NPET) of the large bacterial ribosomal subunit [1]. However, it prevents synthesis of only a limited number of proteins suggesting a novel mechanism of action [1]. |