GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: A-500359 B | antibiotic 446-S3-1
Compound class:
Natural product
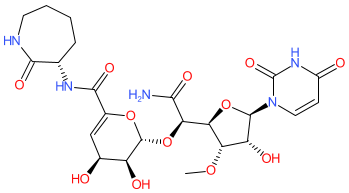
Comment: Capuramycin is a nucleoside antibacterial compound, with activity against Gram-positive bacteria and mycobacteria, and that was initially isolated from the culture filtrate of Streptomyces griseus 446-S3 [5]. It inhibits phospho-N-acetylmuramoyl-pentapeptide-transferase (MraY, translocase I) [3], an essential enzyme in bacterial peptidoglycan biosynthesis.
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. ANDERSON JS, MATSUHASHI M, HASKIN MA, STROMINGER JL. (1965)
LIPID-PHOSPHOACETYLMURAMYL-PENTAPEPTIDE AND LIPID-PHOSPHODISACCHARIDE-PENTAPEPTIDE: PRESUMED MEMBRANE TRANSPORT INTERMEDIATES IN CELL WALL SYNTHESIS. Proc Natl Acad Sci U S A, 53 (4): 881-9. [PMID:14324547] |
|
2. Heydanek Jr MG, Neuhaus FC. (1969)
The initial stage in peptidoglycan synthesis. IV. Solubilization of phospho-N-acetylmuramyl-pentapeptide translocase. Biochemistry, 8 (4): 1474-81. [PMID:5805290] |
|
3. Muramatsu Y, Ishii MM, Inukai M. (2003)
Studies on novel bacterial translocase I inhibitors, A-500359s. II. Biological activities of A-500359 A, C, D and G. J Antibiot (Tokyo), 56 (3): 253-8. [PMID:12760681] |
|
4. STRUVE WG, NEUHAUS FC. (1965)
EVIDENCE FOR AN INITIAL ACCEPTOR OF UDP-NAC-MURAMYL-PENTAPEPTIDE IN THE SYNTHESIS OF BACTERIAL MUCOPEPTIDE. Biochem Biophys Res Commun, 18: 6-12. [PMID:14265759] |
|
5. Yamaguchi H, Sato S, Yoshida S, Takada K, Itoh M, Seto H, Otake N. (1986)
Capuramycin, a new nucleoside antibiotic. Taxonomy, fermentation, isolation and characterization. J Antibiot (Tokyo), 39 (8): 1047-53. [PMID:3759655] |