GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
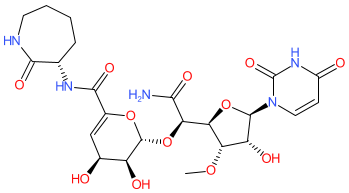
Synonyms: A-500359 B | antibiotic 446-S3-1
Compound class:
Natural product
Comment: Capuramycin is a nucleoside antibacterial compound, with activity against Gram-positive bacteria and mycobacteria, and that was initially isolated from the culture filtrate of Streptomyces griseus 446-S3 [5]. It inhibits phospho-N-acetylmuramoyl-pentapeptide-transferase (MraY, translocase I) [3], an essential enzyme in bacterial peptidoglycan biosynthesis.
|
|
|||||||||||||||||||||||||||||||||||