GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
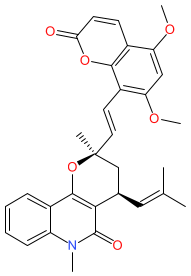
Synonyms: compound 8 [PMID: 24597921]
Compound class:
Natural product
Comment: Toddacoumalone is one of a number of coumarin type compounds identified in extracts from the roots of the African Zanthoxylum (Toddalia) asiatica climbing plant. The plant is used medicinally by many African peoples. Experimental analysis demonstrates that toddacoumalone inhibits phosphodiesterase 4 (PDE4) enzymatic activity [1].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| In vitro, toddacoumalone inhibited PDE4D2 catalysis more potently (IC50 140 nM) than the well-characterized PDE4 inhibitor rolipram (IC50 590 nM) [1] |
| Selectivity at enzymes | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||