GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
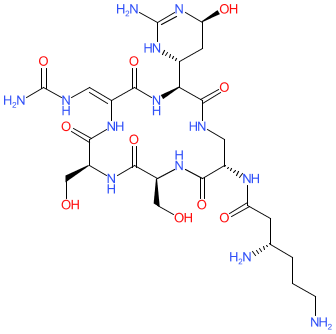
Synonyms: celiomycin | florimycin | tuberactinomycin B
viomycin is an approved drug (FDA (1953))
Compound class:
Natural product
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Viomycin disrupts bacterial protein synthesis by blocking elongation factor G-mediated translocation of mRNA [2,4]. |