GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: espinomycin A | platenomycin B1 | rubimycin | SF-837 | turimycin P3
midecamycin is an approved drug
Compound class:
Natural product
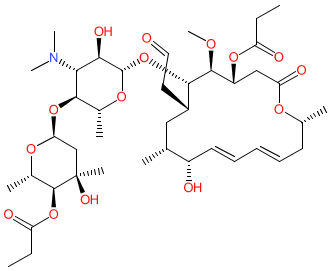
Comment: Midecamycin is a 16-membered macrolide antibacterial, originally isolated from culture broth of Streptomyces mycarofaciens [1-2].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |