GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: NN-2211 | NN2211 | NNC 90-1170 | NNC-90-1170 | Victoza®
liraglutide is an approved drug (EMA (2009), FDA (2010))
Compound class:
Peptide
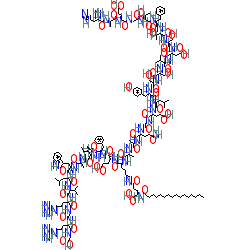
Comment: Liraglutide is a synthetic analog of human GLP-1, with the addition of a palmitic acid moeity which acts to increase the drug's half-life and prolong its activity.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: liraglutide |
|
|||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Liraglutide is already approved to treat type 2 diabetes and it is in development for the treatment of obesity (Saxenda®) [1]. Recent laboratory research in mice suggests that liraglutide may reduce brain inflammation in Alzheimer's disease (AD), leading to an improvement in the growth of brain cells and the connections between them [3-4]. As a result of this repurposing indication, clinical trials are ongoing to investigate the benefit that use of this drug may bring to the prevention of progression of AD in patients with early signs of the disease. Click here to link to the AD trials involving this drug registered with Clinicaltrials.gov. |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |