GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
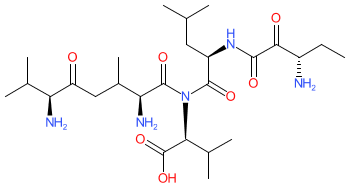
Synonyms: L-Val-L-Val-3-amino-2-oxo-valeryl-D-Leu-L-Val
Compound class:
Natural product
Comment: Poststatin is produced by Streptomyces viridochromogenes MH534-30F3 [1]. It acts as an inhibitor of prolyl endopeptidase.
|
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Natural product |
IUPAC Name  |
| (2S)-2-[[(2R)-2-[[(3S)-3-amino-2-oxopentanoyl]amino]-4-methylpentanoyl]-[(2S,6S)-2,6-diamino-3,7-dimethyl-5-oxooctanoyl]amino]-3-methylbutanoic acid |
Synonyms  |
| L-Val-L-Val-3-amino-2-oxo-valeryl-D-Leu-L-Val |
Database Links  |
|
| Specialist databases | |
Antibiotic DB

|
Poststatin |
| Other databases | |
| CAS Registry No. | 135219-43-1 (source: PubChem) |
| GtoPdb PubChem SID | 405560408 |
| PubChem CID | 164380 |
| Search Google for chemical match using the InChIKey | UNPBSZUDTFBULK-CZCKBYKRSA-N |
| Search Google for chemicals with the same backbone | UNPBSZUDTFBULK |
| UniChem Compound Search for chemical match using the InChIKey | UNPBSZUDTFBULK-CZCKBYKRSA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | UNPBSZUDTFBULK-CZCKBYKRSA-N |