GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: ZP-1848 | ZP1848
Compound class:
Peptide
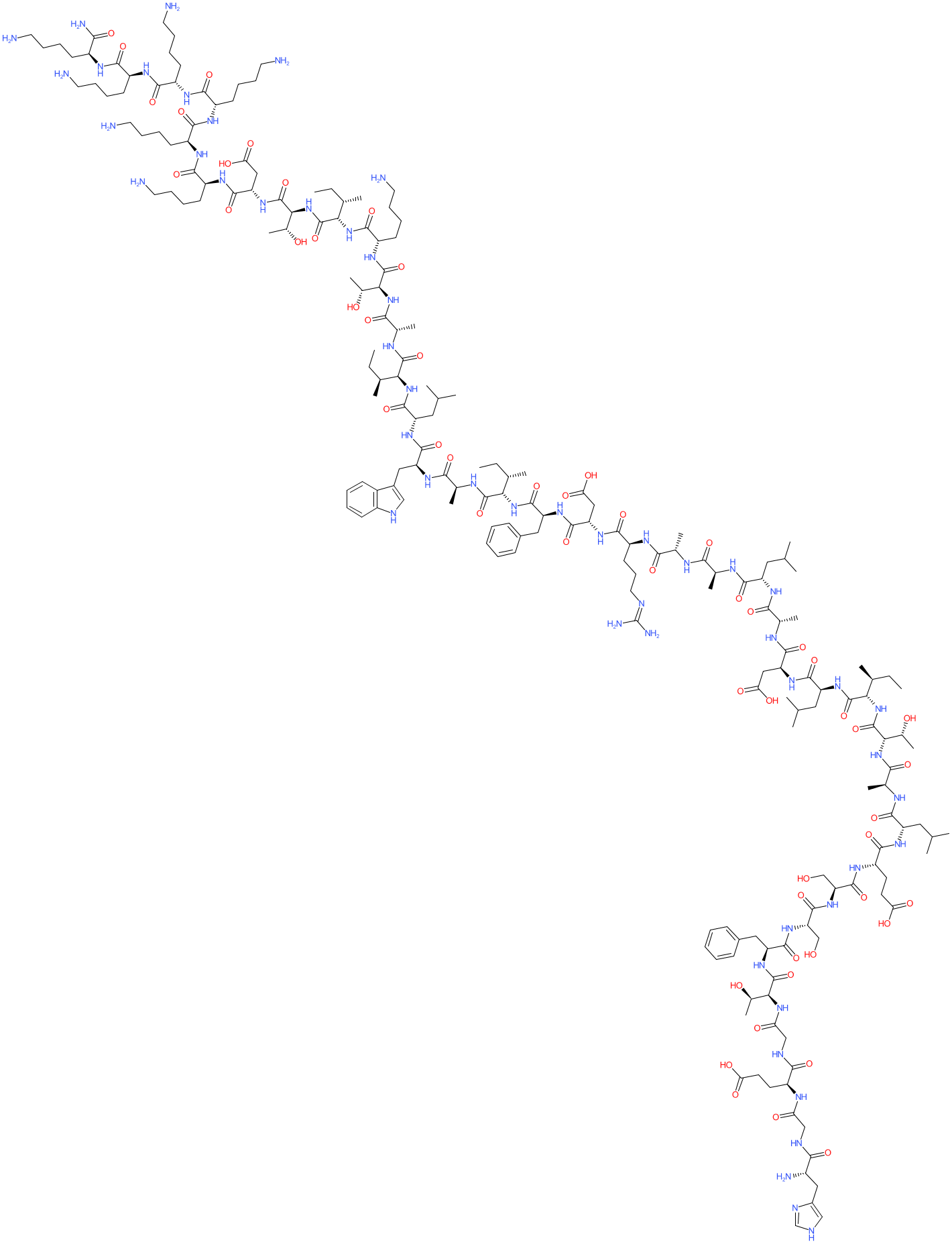
Comment: Glepaglutide (ZP1848, Zealand Pharma) is a long-acting human glucagon like peptide-2 (GLP-2) analogue with a C-terminal hexa-lysine addition.
The wild type peptide sequence is HADGSFSDEMNTILDNLAARDFINWLIQTKITD, compared to the glepaglutide sequence which is HGEGTFSSELATILDALAARDFIAWLIATKITDKKKKKK (the hexa-lysine is underlined). GLP-2 receptor agonists have therapeutic potential in clinical indications with compromised absorptive capacity at the intestinal mucosa, such as in short bowel syndrome. GLP-2 analogues have been designed to be less susceptible to enzymatic cleavage and renal clearance and hence have an improved circulating half-life compared to the endogenous peptide. Teduglutide is already approved but has a once-daily injection regimen, so longer-acting analogues are still of development interest. |
|
|||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Having completed Phase 2 clinical testing in patients with short bowel syndrome [2-3], glepaglutide has been advanced to Phase 3 clinical evaluation. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03690206 | Efficacy And Safety Evaluation of Glepaglutide in Treatment of SBS | Phase 3 Interventional | Zealand Pharma | ||
| NCT03279302 | Trial to Evaluate the PK Profile of Glepaglutide (ZP1848) After a Single IV and After Multiple SC Injections in Healthy Subjects | Phase 1 Interventional | Zealand Pharma | ||
| NCT03905707 | Evaluation of Long Term Safety and Efficacy of Glepaglutide in Treatment of SBS | Phase 3 Interventional | Zealand Pharma | ||