GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Two-pore domain potassium channels (K2P): Introduction
INTRODUCTION TO K2P CHANNELS
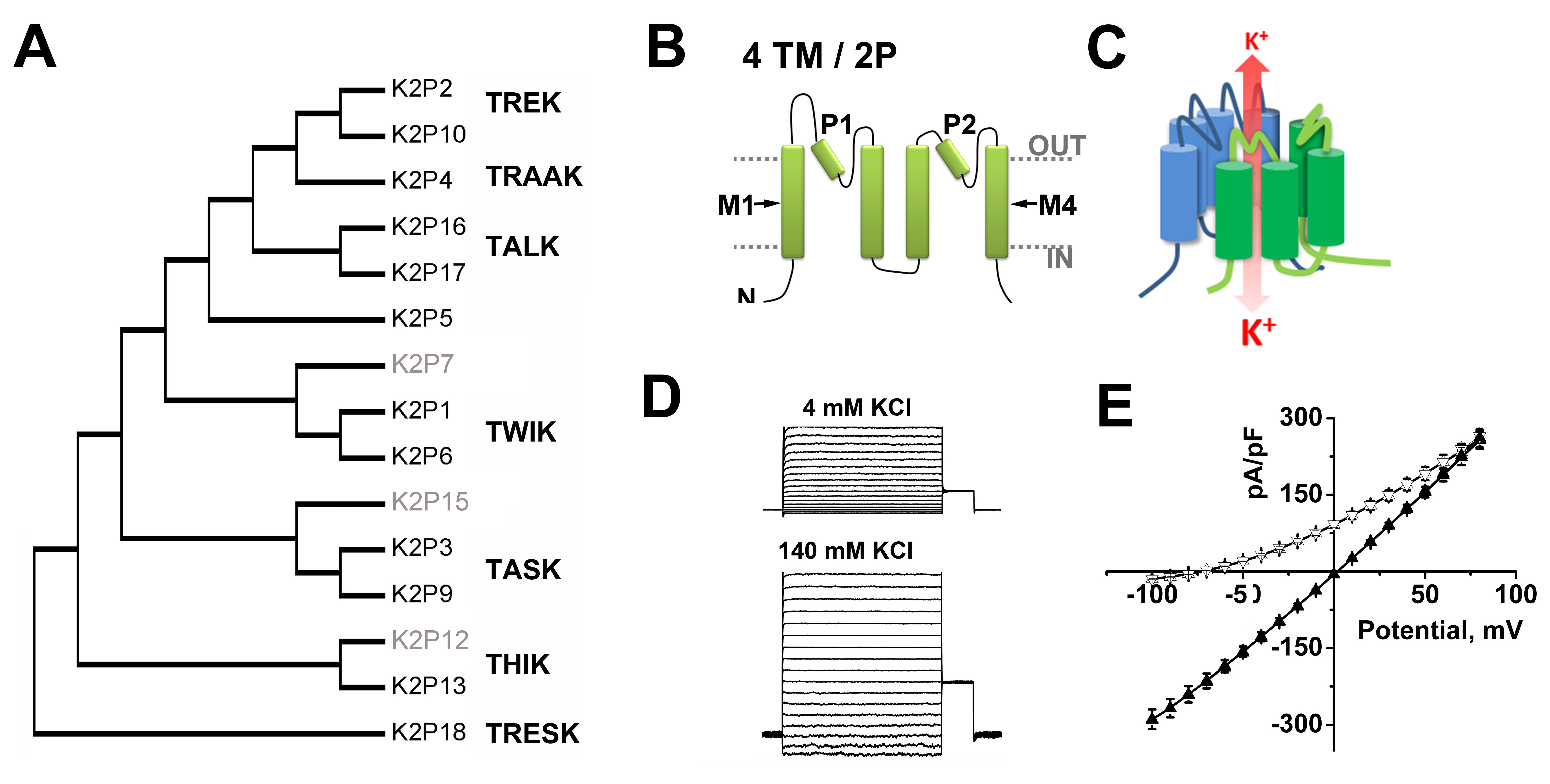
Potassium selective channels are the largest class of mammalian ion channel proteins, with over 75 genes in four distinct families that encode pore-forming subunits- diversity is further enhanced by subunit variation due to alternative splicing, alternative translation initiation (ATI), heteromeric assembly of subunits, and by co-assembly with accessory proteins. To this cavalcade of K+ channels, the two-pore domain (K2P) K+ channel family contributes 15 genes (Figure 1A) encoding proteins notable for their unique subunit architecture, biophysical properties and roles in physiology [53].
K2P channel subunits have a distinct body plan- each has four transmembrane domains (TMD), two re-entrant pore (P)-forming loops, and intracellular amino- and carboxy-termini (Figure 1B). Two identical K2P subunits create a single, central K+ selective conduction pore (Figure 1C) and, in at least some cases, heterodimeric complexes form in vivo [31,105].
The open probability of K2P channels is largely independent of changes in the voltage difference across the plasma membrane (ΔVm) under physiological conditions. Thus, K2P channels are unlike voltage-gated KV channels that show increased open probability (Po) when changes in Vm act on charge-bearing voltage sensor domains (VSDs); inwardly rectifying Kir channels that change Po when changes in ΔVm drive K+ ions through the selectivity filter, unblocking the conduction pore; or calcium-activated Kca channels that respond both via VSDs and by sensing local changes in intracellular calcium. Rather, K2P channels pass K+ currents across the physiological voltage range that are subject to a broad array of regulatory chemical and physical influences [41,43,53,59], including neurotransmitters, post-translation modification (phosphorylation and SUMOylation), all three classes of second messengers (hydrophobic, hydrophilic and gaseous), temperature and mechanical stretch. This was expected [54-55] based on the early descriptions of native K+ background 'leak' currents [58,61,137]. Because K2P channels respond to many of these regulatory influences concurrently, some have been characterized as 'polymodal signal integrators' [6,59,83].
K2P channels are proving to be broadly impactful in the brain, peripheral nervous system, heart and muscles through control of excitability, principally, by setting the level and the stability of cellular resting Vm and the frequency and morphology of action potentials- for example, in hypoglossal motoneurons [12,123], dorsal root ganglion [63,98] and cerebellar granule neurons [103,106]- where they are implicated in thermosensation of both cold and warm, nociception and the action of volatile anesthetics. K2P channels are also manifesting as important in non-excitable cells, including, the pancreas, vascular endothelium, immune T cells and a variety of cancers.
The first K+ channel subunit identified with two-P domains, TOK1, was cloned from the genome of the budding yeast Saccharomyces cerevisiae [65]. TOK1 channels are formed by two identical subunits, each with 8 TMD, and pass outwardly rectifying potassium currents through a single conduction pore. The TOK family of channels appears to be exclusive to fungi based on genomic analyses and is considered in detail elsewhere [53,100]. In 1996, K2P subunits with 4 TMD were identified in the genomes of Drosophila melanogaster, Caenorhabditis elegans [54], and mammals [71].
The fruit fly isolate, K2PΘ (originally called dORK, for Drosophila openly-rectifying K+ channel) was shown to display the functional properties predicted by Hodgkin and Huxley for a canonical background K+ channel [54,58,61,61,137]. K2PΘ channels pass K+ selective currents that show little dependence on voltage or time, and consistent with their operation as Goldman-Hodgkin-Katz (GHK) open rectifiers, the single channels were found to operate as open, multi-ion, selective leak portals, passing ions more readily across the membrane from the side of higher ion concentration. Thus, K2PΘ channels pass larger outward K+ currents than inward because intracellular K+ concentration is high (~140 mM) compared to normal extracellular levels (~4 mM) (Figure 1D). K2PΘ is regulated by changes in the conformation of the K+ selectivity filter induced by phosphorylation of the cytoplasmic C-terminus [6,28,61,137].
Since 1996, fifteen mammalian KCNK genes have been identified in humans that are also present in rats and mice (Figure 1A). Each channel was named as it was cloned to reflect the particular physiological, pharmacological or biophysical attributes judged to be distinctive at the time (Table). The first mammalian K2P subunit was called 'tandem of P-domains in a weak inward rectifying K+ channel', or TWIK1 for short [71]. As differences in channel behavior between experimental reports accrued, the utility of initial phenotypic monikers was diminished, supporting the adoption of formal IUPHAR nomenclature in 2005 [52]. Although proteins encoded by KCNK genes are now called K2P subunits (and are numbered to match their encoding gene, i.e., K2P1 is encoded by KCNK1) the original names remain in use, accompanied by their formal names to keep the literature clear and to speed efforts to correlate channels with the K+ currents they mediate in native cells.
Comparison of K2P channels revealed that a few genes first described in other species were homologs of a human isolate. For this reason, the K2P family contains 15 genes numbered 1-18 and there are no KCNK8, KCNK11 or KCNK14 genes or associated proteins.
Figure 1. Classification of function of two-pore domain channels.
A. A phylogenetic tree calculated to show the relatedness of the 15 K2P subunits found in humans based on ClustalW alignments of the IUPHAR accession numbers for each clone, (see http://www.guidetopharmacology.org/GRAC/IonChannelListForward?class=VGIC). To date, functional expression has not been observed for K2P 7, K2P 12 and K2P 15 (grey text).
B. K2P subunits are integral membrane proteins with internal amino (N) and carboxy (C) termini, four transmembrane domains (M1-M4) and two pore forming (P)-loops.
C. Two K2P subunits create a single, central K+ selective conduction pore. One subunit is shown in green and a second in blue. Under physiological conditions, K+ flow (red arrow) down a concentration gradient from the intra- to the extracellular milieu.
D. Example whole-cell current from K2P1 channels heterologously expressed in CHO-K1 cells and studied with a deSUMOylating enzyme in the recording pipette. In both case, the inside of the cell contains 140 mM KCl. Left, The external solution contains 4 mM KCl. Right, The same cell recorded with 140 mM KCl on both sides of the membrane.
E. Mean current-voltage relationships for Chinese hamster ovary (CHO-K1) cells expressing SENP1 activated K2P1 channels (adapted from [101]). Active K2P1 channels show openly rectifying (GHK) behavior. Under quasi-physiologic conditions (Δ, 4 mM external KCl) the channels pass more outward current but show a linear current-voltage relationship with symmetrical 140 mM KCl (▲). Click image for full size.
| IUPHAR channel name | HUGO gene name | Common name | Other names |
| K2P1 | kcnk1 | TWIK1 | hOHO |
| K2P2 | kcnk2 | TREK1 | TPKC1 |
| K2P3 | kcnk3 | TASK1 | TBAK1, OAT1 |
| K2P4 | kcnk4 | TRAAK | KT4 |
| K2P5 | kcnk5 |
| TASK2 |
| K2P6 | kcnk6 | TWIK2 | TOSS |
| K2P7 | kcnk7 |
| kcnk8 |
| K2P9 | kcnk9 | TASK3 |
|
| K2P10 | kcnk10 | TREK2 |
|
| K2P12 | kcnk12 | THIK2 |
|
| K2P13 | kcnk13 | THIK1 |
|
| K2P15 | kcnk15 | TASK5 | kcnk11, kcnk14 |
| K2P16 | kcnk16 | TALK1 |
|
| K2P17 | kcnk17 | TALK2 | TASK4 |
| K2P18 | kcnk18 | TRESK |
|
Table: The nomenclature of mammalian K2P channels
The 15 mammalian K2P channels are named according to IUPHAR nomenclature and numbered to match the HUGO (Human Genome Organization) designations for each of the genes. Common and Other Names are based on biophysical or pharmacological properties in early reports on each clone. TWIK, tandem of P-domains in a weak inward rectifying K+ channel; TREK, TWIK-related K+ channel; TASK, two-pore domain, acid-sensitive K+ channel; TRAAK, two-pore domain related arachidonic acid activated K+ channel; THIK, two-pore domain halothane inhibited K+ channel; TALK, two-pore domain alkaline activated K+ channel; and TRESK, TWIK-related spinal cord potassium channel. K2P6, 7, 12 and 15 pass little or no current when expressed in heterologous cells and were given common names based on sequence homology.
K2P CHANNELS AT ATOMIC RESOLUTION
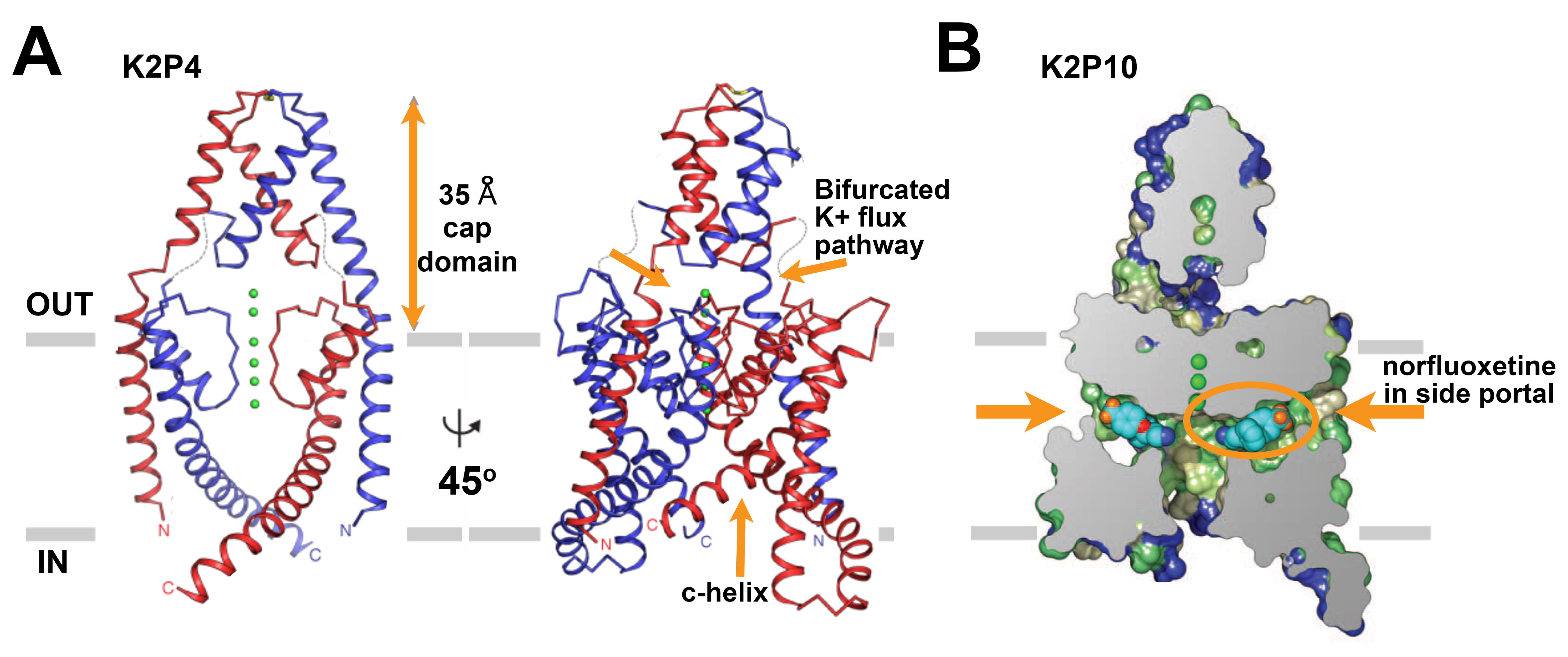
In 2012, X-ray structures were reported for human K2P1 (TWIK1) [86] and K2P4 (TRAAK) [20] (Figure 2A). Each structure was solved at ~3.8 Å with four K+ ions in the conduction pore. As expected, based on prior structure-function analysis and homology models [66,120], the selectivity filter has 4-fold symmetry and is located within a bilaterally symmetric channel corpus. Perhaps the most unexpected feature in both structures was an extracellular ‘cap-domain’ formed by the first external loop of each subunit; the cap-domain extends into the extracellular space ~35 Å above the outer mouth of the pore, creating two pathways for K+ ions to enter or exit the selectivity filter. To date, the cap-domain has not been observed in other K+ channels and appears to offer steric obstruction as an explanation for why classical pore-blocking venom toxin peptides fail to inhibit K2P channels and background K+ currents in native cells. Other interesting features of the structures are two lateral portals that may expose the ion conduction pore to the hydrophobic span of the lipid bilayer and the variability in the end of M4 that connects to a functionally important part of many K2Ps, the C-terminal tail that has been posited to play a role in transmission of gating signals to the selectivity filter [5,17,26,114,137-138]. This key modulatory element has yet to be resolved by any of the K2P structures [18-21,39,74,86] and remains an element of great interest.
In 2013, the structure of K2P4 was refined as a co-crystal with a Fab antibody fragment at 2.75 Å. The higher resolution structure [18,20] revealed that the M1 helix is domain swapped between the two subunits, a topology that was also seen in the structures of two K2P4 gain-of-function mutants [74]and in structures of K2P10 (TREK-2) [39]. The functional consequence of this topology is unclear but may facilitate inter-subunit communication. The cap-domain and the two lateral portals in the membrane spanning region were also observed in crystal structures of a truncated variant of the K2P10 channel [39], suggesting that these features will be held in common throughout the K2P channel family. The structure of K2P10 was solved at 3.9 Å in complex with norfluoxetine, the active metabolite of the selective serotonin reuptake inhibitor Prozac, bound within the lateral portals (Figure 2B). This structure raises interesting questions. Similar to K2P1 and K2P4, the K2P10 structure, was modeled with K+ ions in the conduction pore. However, norfluoxetine is an inhibitor of K2P10 suggesting that the structure is a non-conductive conformation despite the apparently fully occupied selectivity filter. Moreover, a second structure at 3.4 Å, without norfluoxetine but with K+ ions in the conduction pore, shows an alternate arrangement with M4 occluding the two lateral portals and precluding membrane lipid access to the conduction pathway [39]. In all of the structures determined to date, there is an open path to the cytoplasm, consistent with the idea that the principal gate in K2Ps is the selectivity filter [5-6,28,137], rather than an intracellular occlusion made by the pore-lining helices.
Figure 2. The primary structures of the subunits of the voltage-gated sodium channels. A. Ribbon representations of the x-ray structure of human K2P4 resolved at 2.75 Å [18]. Left, The channel is viewed from the membrane plane with one subunit in red and the other in blue, K+ ions are green, and the boundary of the membrane is in grey. Right, A view of the channel rotated by ~45° to show architecture wherein the outer pore helix interacts with the inner helix from the other subunit rather than its own. Dashed lines suggest loop regions that were not resolved. The ~35 Å cap domain above the outer mouth of the pore that bifurcates the entrance to the K+ conduction pathway and the c-helix are indicated with arrows.
B. A cross section of a surface view of human K2P10 colored by hydrophobicity where green is the most hydrophobic and blue is the least hydrophobic, [39]. The structure was solved to 3.9 Å in complex with norfluoxetine bound within both bilateral intramembrane side portals. Norfluoxetine is shown in light blue, dark blue, red, and orange for carbon, nitrogen, oxygen, and fluorine atoms, respectively. Click image for full size.
K2P CHANNEL GATING
Although the open and closed structures of K2P channels, and the nature of the transitions between conformations, remain to be clarified [90], a body of data supports the conclusion that K2P ion conduction is controlled by a “C-type” gate [5-6,28,137]. First described in KV channels, C-type gating refers to suppression of ion permeation due to conformational changes in the selectivity filter, rather than constriction of the conduction pathway below the filter or intracellular pore occlusion by the N-terminus [136]. Occupancy of the K+ binding site external to the filter appears to explain why high bath concentrations of K+ ions stabilize particular open states of K2PΘ [137]. Similarly, heat and stretch appear to augment K2P2 (TREK-1) channel currents in a manner mediated by changes in the activity of a C-type gate [6] and are proposed to stabilize particular open states of the channel [83]. Interestingly, conformational changes in the filter of K2PΘ and K2P2 depend on intracellular regulatory elements, phosphorylation [137] and temperature induced changes in thermal sensing (Ct) domain [5], respectively. A mechanistic basis for the transduction of intracellular signals through the channel corpus is suggested by recent crystallographic findings: K2P4 channels with mutations that activate the C-type gate demonstrate tilting and straightening of M4 and buckling of the M2 helix [74].
Whereas KV channels contain an inner gate formed by ‘bundle-crossing’ helices distinct from the selectivity filter, neither structural nor functional data support the presence of an equivalent restrictive gate in K2P channels [6,18,39,74,99,111,137]. Thus, accessibility of quaternary ammonium ions to the inner pore of K2P2 channels is unchanged by activation, suggesting that the inner portion of the conduction pore is constitutively open [99,111]. Nonetheless, the structures of K2P4 and K2P10 suggest that membrane lipid [20-21] and norfluoxetine [39], respectively, can access the inner vestibule of the channel below the potassium conduction pathway to suppress permeation. Similarly, molecular dynamics simulation (MDS) and mutagenesis suggest that dewetting of the inner pore of K2P1 by the motion of hydrophobic residues may contribute to channel closure [2]. At present, it is unclear how these observations consistent with inner pore occlusion are related to the operation of what appears to be a controlling C-type filer gate, and if they represent mechanisms present throughout the K2P family.
Although K2P channels do not contain a voltage-sensing domain as found in KV channels, intracellular phosphorylation of hippocampal K2P2 channels has been demonstrated to produce voltage dependent operation under physiological conditions [17]. This may relate to a study showing that some K2P channels studied in symmetrical elevated KCl and the absence of activators, open in voltage-dependent manner (ascribed to movement of K+ ions within the selectivity filter) and operate as openly rectifying leak channels once activate [116].
HIGHLIGHTS OF K2P CHANNEL BY SUBTYPE
The operation of K2P channels is subject to tight regulatory control [53]. Thus, their description must highlight the modulatory pathways that act directly on the subunits: the list includes but is not limited to phosphorylation, pH, medications and their metabolites, drugs of abuse, volatile anesthetics, second-messengers, cytoskeletal proteins, post-translational modification pathways and non-covalent assembly with protein-partners. Other regulatory influences may be direct and via interacting proteins include membrane stretch, temperature and oxygen tension. Identification of some pathways that regulate K2P channels have revealed new cellular [104] and molecular [108] pathways for study. K2P regulators change the density of channels at the cell surface, modify open probability, block the pore, alter single channel conductance, and change ion selectivity. Those that decrease K+ flux increase cellular excitability as they permit membrane depolarization whereas stimuli that increase the activity of K2P channels dampen excitability due to membrane hyperpolarization.
Here, we highlight key functional attributes of K2P subunits by the 15 human genes organized according to IUPHAR nomenclature and noting prevalent common names; a more complete fingerprint of each channel is found in the accompanying database while detailed appraisal of the biophysics, structure-function, pathophysiology and pharmacology of each K2P channel can be found in exhaustive reviews [41,53,59,100] and in the citations that accompany each listing in the database.
K2P1 Channels
K2P1 (TWIK1) has been an enigma since it was described as an acid-insensitive weak inward rectifier on heterologous expression [71]; the debate remains vigorous. Several groups reported that they observed no channel activity in the same expression systems [55,107]. Since mRNA transcripts for the KCNK1 gene are abundant in kidney, placenta, lungs, [71,124], and throughout the central nervous system [124], and cardiac conduction pathway [47], it was apparent that the gene was transcribed. Moreover, the protein was observed at the cell surface. This suggested it operated as a receptor that was electrically silent or that a regulator of the channel had yet to be identified. Three mechanisms have been proposed to account for the low basal activity of K2P1 on heterologous expression.
SUMO regulation. In 2005, data were published showing that K2P1 channels at the plasma membrane were held silent when they were covalently modified on Lys274 by the small ubiquitin-like modifier protein, SUMO [108]. SUMOylation is an enzyme mediated, reversible post-translational modification pathway found in all eukaryotic cells [56]. K2P1 channels only passed current when SUMO was removed from the channels with SUMO-specific proteases (SENPs) or when K2P1-Lys274, a site on the intracellular C-helix of the subunits, was mutated to preclude SUMOylation [101]. Once deSUMOylated, K2P1 channels passed open-rectifying, acid-sensitive, K+ selective currents in COS7 cells, CHO-K1 cells and Xenopus oocytes [101,108], (Figure 1E).
The observation that K2P1 channels were SUMOylated was surprising at first because the SUMO pathway had not previously been shown to operate at the plasma membrane. Now, SUMOylation is recognized to regulate the activity of a growing cadre of membrane proteins, including KV channels [11,35,102], TRP channels [67] and kainate receptor ion channels [82]. Yet, questions remain. Some groups failed to observe SUMOylated K2P1 after detergent purification [42], however, the labile nature of SUMO-target conjugates during isolation is known in many cases where target proteins operate as if SUMOylated in the intact cells [56]. Indeed, both fluorescent resonance energy transfer (FRET) and single molecule visualization using total internal reflection microscopy were subsequently used to verify assembly of K2P1 and SUMO1 in live cells [101,105].
Hydrophobic dewetting. Based on the crystal structure of human K2P1 [86], molecular dynamic simulations (MDS) identified a “hydrophobic cuff” in the inner vestibule of the channel below the selectivity filter comprised of four residues, Leu146 on M2 and Leu261 on M4, from each subunit [2]. One hundred ns simulations showed stochastic motion of the cuff to restrict water access to the internal entrance of the pore, creating an energetic barrier to ion permeation. In support of this model, hydrophilic mutations of Leu146 were reported to yield K2P1 channels currents in Xenopus oocytes that were not observed with wild type channels [2,23].
Rapid endocytosis. Others attribute the low basal activity of K2P1 on heterologous expression to rapid, constitutive trafficking of the channel away from the plasma membrane to recycling endosomes [13,44]. Site-directed mutagenesis showed that endocytosis was dynamin dependent and required a di-isoleucine motif (Ile293Ile294) in K2P1 [44]. Mutation of two residues yielded channels that passed measurable currents in MDCK cells, HEK293 cells and Xenopus oocytes. The channel was also reported to interact with ARF6, a small G protein that modulates endocytosis at the apical surface of epithelial cells, and forms complexes with the nucleotide exchange factor EFA6 [37]. Indeed, endocytosis has also been implicated in the surface half-life of K2P2, K2P3, K2P9 and K2P18, and in the cases of K2P1 and K2P3, both clathrin-mediated and clathrin-independent mechanisms appear to operate [93]. How SUMOylation, hydrophobic dewetting of the inner pore, and endocytosis contribute individually or in concert to regulation of K2P1 in native cells remains under active study.
While K2P1 has yielded measurable currents in experimental expression systems only after enzyme treatment or point mutation, evidence continues to accrue that the native channels are active and play key roles in plasma membrane excitability in several tissues. Thus, mice deficient in K2P1 show altered Vm in pancreatic β cells [23] and manifest defects in renal transport of phosphate and water in their proximal tubules and medullary collecting ducts, respectively [88]. Using transgenic zebrafish, K2P1 was demonstrated to control heart rate as well as atrial morphology [27]. In rat cerebellar granule neurons (CGN), K2P1 subunits were shown to pass IKso, the standing outward, background K+ current that determines Vm and excitability of CGN (via assembly with K2P3 or K2P9 to form heterodimeric channels) [105]. IKso determines the response of these neurons to changes in pH [85], pO2 [103], and volatile anesthetics subject to regulation by SUMO of K2P1 [105]. In humans, K2P1 mediates arrhythmogenic depolarization of myocytes exposed to low serum potassium (hypokalemia), an anomalous response the authors showed was due to enhanced permeation of the channels by Na+ [51,79]. Supporting this unexpected behavior for a K+ selective channel, K2P2 had previously been shown in rat brain in two forms due to ATI, one manifesting a stable change in ion selectivity allowing Na+ flux [125].
Whereas K2P1 does not appear to assemble with K2P2 in rat CGN [105], the two subunits were proposed to form dimers in hippocampal astrocytes yielding a passive membrane K+ conductance that set Vm [133]. However, this finding has also proven to be controversial. Subsequent studies suggested that K2P1 [133] and K2P2 were retained inside astrocytes and that the electrical properties were unaltered in astrocytes from mice lacking both channels [40]. Yet another study found trafficking of K2P1 and K2P2 to the astrocyte surface to be interdependent, and that K2P1-K2P2 heterodimers were essential for passive K+ conductance and cannabinoid-induced glutamate release [60]. Indeed, glutamate has been proposed to play a role in forward trafficking of K2P1 via a metabotropic glutamate receptor pathway [132]. Perhaps, K2P1 trafficking and assembly with K2P2 will prove to be differentially regulated in a cell-type dependent fashion.
K2P2, K2P4 and K2P10 channels
Ready expression of K2P2 (TREK1, TWIK-related K+ channel 1; Table), the closely related K2P4 (TRAAK, TWIK-related arachidonic acid activated K+ channel) and K2P10 (TREK2) channels has expedited their study. All three are expressed throughout the CNS and the myocardium [45,84,105,124,133], and are subject to regulation by a diverse array of regulators, including lipids, neurotransmitter-activated second messenger pathways, phosphorylation, anesthetics and drugs [17,39,59]. This subgroup of K2P channels is also subject to regulation by physical stimuli, including temperature [1,92] and mechanical stretch [20,80-81]. Because physical stimuli are often coincident in biology, this group of channels are proposed to act as polymodal signal integrators [59].
The importance of K2P2 and K2P4 as thermal sensors in native cells remains a matter of controversy because they are ~5 times less sensitive to temperature than TRPV channels [1,62]. Thus, knock-out mice lacking Kcnk2 and Kcnk4 genes show altered responses to changes in temperature [92] but some posit this is due to close localization of K2P2 and TRP channels in the native cells [135], allowing K2P to tune the temperature response indirectly via regulation of neuronal excitability. However, arguing otherwise, the intracellular C-terminus of K2P2 has been shown to operate directly as a primary heat-sensing element in the channel with a strong mechanistic rationale for linkage to the C-type gate in the filter [5].
The classification of K2P2 and K2P4 as mechanosensitive channels [80-81] rather than secondarily responsive to the stimuli due to changes to the fluidity and composition of the plasma membrane [41,59] has also generated disagreement. Perhaps, these controversies will prove to be semantic. All ion channels show varying degrees of responsiveness to temperature and mechanical stress; for some this is a critical defining feature, with well defined structural determinants, whereas for other channels of lower responsiveness the impact on physiology may be limited or non-essential.
The effort to correlate native currents and K2P2 and K2P4 channels is ongoing. In addition to polymodal regulation, both K2P2 [125] and K2P10 [118] are subject to ATI, a process whereby a downstream start codon is used to generate a foreshortened version of each subunit; for K2P2, ATI is regulated in a developmentally and spatially determined manner in rat brain to yield a channel that allows increased Na+ ion permeability. Suggesting a role for K2P2 in nociception and analgesia, and an in vivo effect of ATI, K2P2 current is reversibly increased by exposure to fenamate-derived non-steroid anti-inflammatory agents [128], and while augmentation is not yet understood in full-length channels, the effect on the ATI-truncated K2P2 channels appears to be due to an increase in K+ selectivity. Also of note, the truncated ATI isoform is insensitive to diclofenac, the most commonly prescribed fenamate derivative in the United States [128].
Diversity of K2P2 function arises as well through protein kinase A-dependent phosphorylation of Ser348, a modification that endows the channels with voltage-dependence when expressed in tissue culture cells and natively in hippocampal cells [17]. Further, increased intracellular PIP2 shifts the voltage-dependence of K2P2 channels to hyperpolarized potentials, augmenting current magnitude across the physiological voltage range [25,76]. Finally, recent reports suggest that, like K2P1, the operation of K2P2 and K2P4 channels can be varied through formation of heterodimers in both heterologous expression systems [14,72] and in dorsal root ganglion neurons [70].
K2P3 and K2P9 Channels
K2P3 (TASK1, Two P domain, acid-sensitive potassium channel 1), and K2P9 (TASK3) channels are expressed throughout the central and peripheral nervous systems and are proposed to mediate currents that depolarize neurons during acidification [30,105,123]. K2P3 is also expressed in human atrial myocytes and has been shown to mediate increased background K+ current that contribute to the shortening of action potential duration in patients with atrial fibrillation [117]. Levels of mRNA encoding K2P9 are amplified in human breast cancer cells, leading to the proposal that KCNK9 operates as a proto-oncogene [87,97]. Knockout studies in mice indicate that K2P3 and K2P9 are required for adrenal gland development and regulated aldosterone secretion [7,57]. Missense mutations in the maternal copy of the imprinted KCNK9 gene lead to Birk Barel syndrome, presenting in patients as mental retardation, hypotonia, and facial dysmorphism [8].
Like K2P1, K2P3 and K2P9 pass K+ selective currents that are blocked by protonation of a histidine residue in the outer mouth of the pore in the first P loop (Gly-Tyr-Gly-His) [75,77]. In addition to forming SUMO-sensitive heterodimers with K2P1 subunits, K2P3 and K2P9 heterodimerize in vivo to form a channel with distinct sensitivities to acidification [12,31,105] and to pungent stimuli such as hydroxy-α-sanshool, an active ingredient in Szechuan peppers [10].
Surface levels of K2P3 and K2P9 are regulated by the competing actions of protein partners that bind to trafficking motifs on the channel subunit to liberate or retain the channel in the endoplasmic reticulum (ER) as well as by trafficking pathways that operate at the plasma membrane [37]. Forward trafficking to the plasma membrane requires that the channel subunits bind the soluble adapter protein 14-3-3β, in a phosphorylation dependent manner, and this suppresses the interaction with βCOP, a vesicular transport protein that acts to retain the channel in the ER [94,139]. The annexin protein p11 [50], present in some cell types, has also been found to modulate K2P3 trafficking via this pathway, binding in a 14-3-3 dependent manner [95].
K2P5, K2P16 and K2P17 Channels
K2P5 (for a period TASK2), K2P16 (TALK1) and K2P17 (TALK2) pass larger currents as the extracellular pH becomes more alkaline [36,49]. The pH-dependent gating of the channels appears to arise from neutralization of basic residues near the second P loop in the primary structure that influence operation of the selectivity filter (Arg in K2P5 and K2P16; Lys in K2P17). Studies with concatemeric K2P5 subunits show the pH sensors in both subunits must be neutralized for a dimeric channel to conduct K+ ions [91]. Alkaline activation of these channels is proposed to play a role in secretion of biocarbonate in the lumen of the exocrine pancreas [46]. The knock-out of K2P5 in mice has been explicitly associated with bicarbonate handling in proximal tubule cells of the kidney leading to impaired reabsorption, metabolic acidosis, hyponatremia and hypotension [134]. Supporting the relevance of these findings to human disease, Balkan Endemic Nephropathy, a chronic tubulointerstitial disease leading to renal failure, has been associated with a KCNK5 variant (T108P) [126] that produces both loss-of-function [129] and dominant negative effects on K2P5 channels expressed in heterologous systems [112]. K2P5 also appears to contribute to rapid control of acid-base balance by regulating the activity of central respiratory chemoreceptors and CO2 elimination by ventilation [48,131].
K2P5 has also been implicated in cytosolic volume regulation in Ehrlich and mouse proximal tubule cells where increased intracellular pressure yields efflux of K+, along with Cl- and organic osmolytes, obliging outflow of water and normalization of cell volume [9,89]. Moreover, auditory function is lost in K2P5 knockout mice, perhaps because the channels, expressed in the cochlea, spiral ganglion, Reissner's membrane and stria vascularis, are required for K+ recycling in the outer sulcus lateralis [22].
K2P18 Channels
K2P18 (TRESK), the final member of the family to be identified in the human genome [115], also operates as an open rectifying K+ selective channel [10,38,64]. K2P18 is notable for its expression in the spinal cord, trigeminal and dorsal root ganglia where it appears to pass a significant portion of the background K+ current that determines the excitability of somatosensory nociceptive fibers. K2P18 has also been implicated in normal operation of peripheral nociceptor neurons by a ~50% decrease in its mRNA upon axiotomy [127]. Correlation of K2P18 channels and pain was provided by linkage of a frame-shift mutation (F139WfsX24) in KCNK18 and migraine with aura in a large, multigenerational family [68]. The F139WfsX24 variant is truncated at residue 162 and appears to act as a dominant-negative subunit to suppress the function of wild type K2P18 channels. K2P18 is also notable for being reversibly activated by the calmodulin-dependent protein phosphatase calcineurin in response to increased levels of cytosolic Ca2+ [32-33]. Of note, Ca2+ modulation of K2P18 is suppressed by phosphorylation of Ser264 and subsequent binding of 14-3-3η or 14-3-3γ (but not 14-3-3β) to a residue on the intracellular loop of the channel [34].
K2P6, K2P7, and K2P15: subunits that, thus far, remain quiet
K2P6 (TWIK2) and K2P7 exhibit small currents on heterologous expression slowing their characterization and delineation of their roles [16,24]. Both proteins are expressed in the eye, lung and stomach [113]. K2P6 has been posited to play a role in regulating arterial blood pressure by determining the Vm of vascular smooth muscle cells [73]. K2P7 has a His in the first P-domain (Gly-Tyr-Gly-His) that has been proposed to mediate proton block based observations of this role for the homologous residue in K2P1, K2P3 and K2P9 [75,101,110]. In contrast, K2P7 is notable for an unconventional signature sequence in the second P-domain, Gly-Leu-Glu, not found in other human K2P subunits that carry Gly-Tyr-Gly, Gly-Leu-Gly, or Gly-Phe-Gly. The presence of a large, negatively-charged glutamate residue in the second P-domain, rather than a small glycine, has led some to suggest that K2P7 may not function as a K+ channel [41]. K2P15 transcripts are widely expressed in humans but it has not been reported to pass current and has been postulated to require an as yet unidentified subunit or regulator [3].
K2P12 and K2P13 Channels
K2P12 (THIK2) was initially reported to traffic to the cell surface but not to pass current nor to assemble with K2P13 (THIK1) with which it shares sequence similarity [109]; in contrast, K2P13 passed current and was inhibited by the volatile anesthetic halothane. Subsequently, a study reported that encoding K2P12 linked to K2P13 to force their co-assembly as a heterodimeric concatemer produced electrically active channels at the cell surface [15]. Because homodimeric concatemers of K2P12 appeared to be retained in the ER of MDCK cells, the heterodimeric constructs were proposed to mask ER retention or retrieval motifs [15].
THE PHARMACOLOGY OF K2P CHANNELS
To date, K2P channels have few recognized natural blockers and a scant pharmacopeia [78,130]. The lack of identified pore-blocking peptide neurotoxins appears to reflect shielding of the outer vestibule by the cap-domain. Medications acting on K2P channels include the halogenated volatile anesthetics, a class of drugs known to act on background K+ channels before the genes were cloned. Thus, currents mediated by K2P2, K2P3, K2P4, K2P9, K2P10 and K2P18 are augmented by halothane, isofluorane and sevofluorane [69,105,119,122]. In contrast, these same agents suppress K2P13 activity. Although halothane has little impact on the activity of K2P1 channels in CGN, halothane augments the current passed by heterodimeric channels formed by K2P1 and K2P9 by ~300% [105]. Structure-function analysis has revealed that the effect of volatile anesthetics on K2P2, K2P3 and K2P9 channels requires a domain on the proximal C-terminus of the subunits and has implicated direct binding of the Gαq signaling protein [96,122]. Because Gαq proteins diminish K2P channel function, the volatile anesthetics may augment activity by suppressing this interaction [29]. As noted above, K2P10 is inhibited by active metabolite of the selective serotonin reuptake inhibitor Prozac [39] and K2P2 is activated by fenamate-derived anti-inflammatory agents [128]. Efforts are ongoing to develop K2P pharmacology and an activator selective for the mechanosensitive subclass K2P2/K2P4/K2P10 [4,130] and an inhibitor that acts on K2P4, K2P6, and K2P9 [121] have recently been reported.
References
1. Alloui A, Zimmermann K, Mamet J, Duprat F, Noël J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat-Coudert C et al.. (2006) TREK-1, a K+ channel involved in polymodal pain perception. EMBO J, 25 (11): 2368-76. [PMID:16675954]
2. Aryal P, Abd-Wahab F, Bucci G, Sansom MS, Tucker SJ. (2014) A hydrophobic barrier deep within the inner pore of the TWIK-1 K2P potassium channel. Nat Commun, 5: 4377. [PMID:25001086]
3. Ashmole I, Goodwin PA, Stanfield PR. (2001) TASK-5, a novel member of the tandem pore K+ channel family. Pflugers Arch, 442 (6): 828-33. [PMID:11680614]
4. Bagriantsev SN, Ang KH, Gallardo-Godoy A, Clark KA, Arkin MR, Renslo AR, Minor Jr DL. (2013) A high-throughput functional screen identifies small molecule regulators of temperature- and mechano-sensitive K2P channels. ACS Chem Biol, 8 (8): 1841-51. [PMID:23738709]
5. Bagriantsev SN, Clark KA, Minor Jr DL. (2012) Metabolic and thermal stimuli control K(2P)2.1 (TREK-1) through modular sensory and gating domains. EMBO J, 31 (15): 3297-308. [PMID:22728824]
6. Bagriantsev SN, Peyronnet R, Clark KA, Honoré E, Minor Jr DL. (2011) Multiple modalities converge on a common gate to control K2P channel function. EMBO J, 30 (17): 3594-606. [PMID:21765396]
7. Bandulik S, Tauber P, Lalli E, Barhanin J, Warth R. (2015) Two-pore domain potassium channels in the adrenal cortex. Pflugers Arch, 467 (5): 1027-42. [PMID:25339223]
8. Barel O, Shalev SA, Ofir R, Cohen A, Zlotogora J, Shorer Z, Mazor G, Finer G, Khateeb S, Zilberberg N et al.. (2008) Maternally inherited Birk Barel mental retardation dysmorphism syndrome caused by a mutation in the genomically imprinted potassium channel KCNK9. Am J Hum Genet, 83 (2): 193-9. [PMID:18678320]
9. Barriere H, Belfodil R, Rubera I, Tauc M, Lesage F, Poujeol C, Guy N, Barhanin J, Poujeol P. (2003) Role of TASK2 potassium channels regarding volume regulation in primary cultures of mouse proximal tubules. J Gen Physiol, 122 (2): 177-90. [PMID:12860925]
10. Bautista DM, Sigal YM, Milstein AD, Garrison JL, Zorn JA, Tsuruda PR, Nicoll RA, Julius D. (2008) Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat Neurosci, 11 (7): 772-9. [PMID:18568022]
11. Benson MD, Li QJ, Kieckhafer K, Dudek D, Whorton MR, Sunahara RK, Iñiguez-Lluhí JA, Martens JR. (2007) SUMO modification regulates inactivation of the voltage-gated potassium channel Kv1.5. Proc Natl Acad Sci U S A, 104 (6): 1805-10. [PMID:17261810]
12. Berg AP, Talley EM, Manger JP, Bayliss DA. (2004) Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci, 24 (30): 6693-702. [PMID:15282272]
13. Bichet D, Blin S, Feliciangeli S, Chatelain FC, Bobak N, Lesage F. (2015) Silent but not dumb: how cellular trafficking and pore gating modulate expression of TWIK1 and THIK2. Pflugers Arch, 467 (5): 1121-31. [PMID:25339226]
14. Blin S, Ben Soussia I, Kim EJ, Brau F, Kang D, Lesage F, Bichet D. (2016) Mixing and matching TREK/TRAAK subunits generate heterodimeric K2P channels with unique properties. Proc Natl Acad Sci USA, 113 (15): 4200-5. [PMID:27035965]
15. Blin S, Chatelain FC, Feliciangeli S, Kang D, Lesage F, Bichet D. (2014) Tandem pore domain halothane-inhibited K+ channel subunits THIK1 and THIK2 assemble and form active channels. J Biol Chem, 289 (41): 28202-12. [PMID:25148687]
16. Bockenhauer D, Nimmakayalu MA, Ward DC, Goldstein SA, Gallagher PG. (2000) Genomic organization and chromosomal localization of the murine 2 P domain potassium channel gene Kcnk8: conservation of gene structure in 2 P domain potassium channels. Gene, 261 (2): 365-72. [PMID:11167025]
17. Bockenhauer D, Zilberberg N, Goldstein SA. (2001) KCNK2: reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat Neurosci, 4 (5): 486-91. [PMID:11319556]
18. Brohawn SG, Campbell EB, MacKinnon R. (2013) Domain-swapped chain connectivity and gated membrane access in a Fab-mediated crystal of the human TRAAK K+ channel. Proc Natl Acad Sci U S A, 110 (6): 2129-34. [PMID:23341632]
19. Brohawn SG, Campbell EB, MacKinnon R. (2014) Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature, 516 (7529): 126-30. [PMID:25471887]
20. Brohawn SG, del Mármol J, MacKinnon R. (2012) Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science, 335 (6067): 436-41. [PMID:22282805]
21. Brohawn SG, Su Z, MacKinnon R. (2014) Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc Natl Acad Sci U S A, 111 (9): 3614-9. [PMID:24550493]
22. Cazals Y, Bévengut M, Zanella S, Brocard F, Barhanin J, Gestreau C. (2015) KCNK5 channels mostly expressed in cochlear outer sulcus cells are indispensable for hearing. Nat Commun, 6: 8780. [PMID:26549439]
23. Chatelain FC, Bichet D, Douguet D, Feliciangeli S, Bendahhou S, Reichold M, Warth R, Barhanin J, Lesage F. (2012) TWIK1, a unique background channel with variable ion selectivity. Proc Natl Acad Sci U S A, 109 (14): 5499-504. [PMID:22431633]
24. Chavez RA, Gray AT, Zhao BB, Kindler CH, Mazurek MJ, Mehta Y, Forsayeth JR, Yost CS. (1999) TWIK-2, a new weak inward rectifying member of the tandem pore domain potassium channel family. J Biol Chem, 274 (12): 7887-92. [PMID:10075682]
25. Chemin J, Patel AJ, Duprat F, Lauritzen I, Lazdunski M, Honoré E. (2005) A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J, 24 (1): 44-53. [PMID:15577940]
26. Chemin J, Patel AJ, Duprat F, Sachs F, Lazdunski M, Honore E. (2007) Up- and down-regulation of the mechano-gated K(2P) channel TREK-1 by PIP (2) and other membrane phospholipids. Pflugers Arch, 455 (1): 97-103. [PMID:17384962]
27. Christensen AH, Chatelain FC, Huttner IG, Olesen MS, Soka M, Feliciangeli S, Horvat C, Santiago CF, Vandenberg JI, Schmitt N et al.. (2016) The two-pore domain potassium channel, TWIK-1, has a role in the regulation of heart rate and atrial size. J Mol Cell Cardiol, 97: 24-35. [PMID:27103460]
28. Cohen A, Ben-Abu Y, Hen S, Zilberberg N. (2008) A novel mechanism for human K2P2.1 channel gating. Facilitation of C-type gating by protonation of extracellular histidine residues. J Biol Chem, 283 (28): 19448-55. [PMID:18474599]
29. Conway KE, Cotten JF. (2012) Covalent modification of a volatile anesthetic regulatory site activates TASK-3 (KCNK9) tandem-pore potassium channels. Mol Pharmacol, 81 (3): 393-400. [PMID:22147752]
30. Cooper BY, Johnson RD, Rau KK. (2004) Characterization and function of TWIK-related acid sensing K+ channels in a rat nociceptive cell. Neuroscience, 129 (1): 209-24. [PMID:15489043]
31. Czirják G, Enyedi P. (2002) Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J Biol Chem, 277 (7): 5426-32. [PMID:11733509]
32. Czirják G, Enyedi P. (2006) Targeting of calcineurin to an NFAT-like docking site is required for the calcium-dependent activation of the background K+ channel, TRESK. J Biol Chem, 281 (21): 14677-82. [PMID:16569637]
33. Czirják G, Tóth ZE, Enyedi P. (2004) The two-pore domain K+ channel, TRESK, is activated by the cytoplasmic calcium signal through calcineurin. J Biol Chem, 279 (18): 18550-8. [PMID:14981085]
34. Czirják G, Vuity D, Enyedi P. (2008) Phosphorylation-dependent binding of 14-3-3 proteins controls TRESK regulation. J Biol Chem, 283 (23): 15672-80. [PMID:18397886]
35. Dai XQ, Kolic J, Marchi P, Sipione S, Macdonald PE. (2009) SUMOylation regulates Kv2.1 and modulates pancreatic beta-cell excitability. J Cell Sci, 122 (Pt 6): 775-9. [PMID:19223394]
36. Decher N, Maier M, Dittrich W, Gassenhuber J, Brüggemann A, Busch AE, Steinmeyer K. (2001) Characterization of TASK-4, a novel member of the pH-sensitive, two-pore domain potassium channel family. FEBS Lett, 492 (1-2): 84-9. [PMID:11248242]
37. Decressac S, Franco M, Bendahhou S, Warth R, Knauer S, Barhanin J, Lazdunski M, Lesage F. (2004) ARF6-dependent interaction of the TWIK1 K+ channel with EFA6, a GDP/GTP exchange factor for ARF6. EMBO Rep, 5 (12): 1171-5. [PMID:15540117]
38. Dobler T, Springauf A, Tovornik S, Weber M, Schmitt A, Sedlmeier R, Wischmeyer E, Döring F. (2007) TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in murine dorsal root ganglion neurones. J Physiol, 585 (Pt 3): 867-79. [PMID:17962323]
39. Dong YY, Pike AC, Mackenzie A, McClenaghan C, Aryal P, Dong L, Quigley A, Grieben M, Goubin S, Mukhopadhyay S et al.. (2015) K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science, 347 (6227): 1256-9. [PMID:25766236]
40. Du Y, Kiyoshi CM, Wang Q, Wang W, Ma B, Alford CC, Zhong S, Wan Q, Chen H, Lloyd EE et al.. (2016) Genetic Deletion of TREK-1 or TWIK-1/TREK-1 Potassium Channels does not Alter the Basic Electrophysiological Properties of Mature Hippocampal Astrocytes In Situ. Front Cell Neurosci, 10: 13. [PMID:26869883]
41. Enyedi P, Czirják G. (2010) Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev, 90 (2): 559-605. [PMID:20393194]
42. Feliciangeli S, Bendahhou S, Sandoz G, Gounon P, Reichold M, Warth R, Lazdunski M, Barhanin J, Lesage F. (2007) Does sumoylation control K2P1/TWIK1 background K+ channels?. Cell, 130 (3): 563-9. [PMID:17693262]
43. Feliciangeli S, Chatelain FC, Bichet D, Lesage F. (2015) The family of K2P channels: salient structural and functional properties. J Physiol (Lond.), 593 (12): 2587-603. [PMID:25530075]
44. Feliciangeli S, Tardy MP, Sandoz G, Chatelain FC, Warth R, Barhanin J, Bendahhou S, Lesage F. (2010) Potassium channel silencing by constitutive endocytosis and intracellular sequestration. J Biol Chem, 285 (7): 4798-805. [PMID:19959478]
45. Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. (1996) Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J, 15 (24): 6854-62. [PMID:9003761]
46. Fong P, Argent BE, Guggino WB, Gray MA. (2003) Characterization of vectorial chloride transport pathways in the human pancreatic duct adenocarcinoma cell line HPAF. Am J Physiol Cell Physiol, 285 (2): C433-45. [PMID:12711595]
47. Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, Demolombe S. (2007) Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol, 582 (Pt 2): 675-93. [PMID:17478540]
48. Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J et al.. (2010) Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci U S A, 107 (5): 2325-30. [PMID:20133877]
49. Girard C, Duprat F, Terrenoire C, Tinel N, Fosset M, Romey G, Lazdunski M, Lesage F. (2001) Genomic and functional characteristics of novel human pancreatic 2P domain K(+) channels. Biochem Biophys Res Commun, 282 (1): 249-56. [PMID:11263999]
50. Girard C, Tinel N, Terrenoire C, Romey G, Lazdunski M, Borsotto M. (2002) p11, an annexin II subunit, an auxiliary protein associated with the background K+ channel, TASK-1. EMBO J, 21 (17): 4439-48. [PMID:12198146]
51. Goldstein SA. (2011) K2P potassium channels, mysterious and paradoxically exciting. Sci Signal, 4 (184): pe35. [PMID:21868351]
52. Goldstein SA, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. (2005) International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol Rev, 57 (4): 527-40. [PMID:16382106]
53. Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. (2001) Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci, 2 (3): 175-84. [PMID:11256078]
54. Goldstein SA, Price LA, Rosenthal DN, Pausch MH. (1996) ORK1, a potassium-selective leak channel with two pore domains cloned from Drosophila melanogaster by expression in Saccharomyces cerevisiae. Proc Natl Acad Sci USA, 93 (23): 13256-61. [PMID:8917578]
55. Goldstein SA, Wang KW, Ilan N, Pausch MH. (1998) Sequence and function of the two P domain potassium channels: implications of an emerging superfamily. J Mol Med, 76 (1): 13-20. [PMID:9462864]
56. Hay RT. (2005) SUMO: a history of modification. Mol Cell, 18 (1): 1-12. [PMID:15808504]
57. Heitzmann D, Derand R, Jungbauer S, Bandulik S, Sterner C, Schweda F, El Wakil A, Lalli E, Guy N, Mengual R et al.. (2008) Invalidation of TASK1 potassium channels disrupts adrenal gland zonation and mineralocorticoid homeostasis. EMBO J, 27 (1): 179-87. [PMID:18034154]
58. HODGKIN AL, HUXLEY AF. (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol (Lond.), 117 (4): 500-44. [PMID:12991237]
59. Honoré E. (2007) The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci, 8 (4): 251-61. [PMID:17375039]
60. Hwang EM, Kim E, Yarishkin O, Woo DH, Han KS, Park N, Bae Y, Woo J, Kim D, Park M et al.. (2014) A disulphide-linked heterodimer of TWIK-1 and TREK-1 mediates passive conductance in astrocytes. Nat Commun, 5: 3227. [PMID:24496152]
61. Ilan N, Goldstein SA. (2001) Kcnkø: single, cloned potassium leak channels are multi-ion pores. Biophys J, 80 (1): 241-53. [PMID:11159398]
62. Kang D, Choe C, Kim D. (2005) Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol (Lond.), 564 (Pt 1): 103-16. [PMID:15677687]
63. Kang D, Kim D. (2006) TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol, 291 (1): C138-46. [PMID:16495368]
64. Keshavaprasad B, Liu C, Au JD, Kindler CH, Cotten JF, Yost CS. (2005) Species-specific differences in response to anesthetics and other modulators by the K2P channel TRESK. Anesth Analg, 101 (4): 1042-9, table of contents. [PMID:16192517]
65. Ketchum KA, Joiner WJ, Sellers AJ, Kaczmarek LK, Goldstein SA. (1995) A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature, 376 (6542): 690-5. [PMID:7651518]
66. Kollewe A, Lau AY, Sullivan A, Roux B, Goldstein SA. (2009) A structural model for K2P potassium channels based on 23 pairs of interacting sites and continuum electrostatics. J Gen Physiol, 134 (1): 53-68. [PMID:19564427]
67. Kruse M, Schulze-Bahr E, Corfield V, Beckmann A, Stallmeyer B, Kurtbay G, Ohmert I, Schulze-Bahr E, Brink P, Pongs O. (2009) Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest, 119 (9): 2737-44. [PMID:19726882]
68. Lafrenière RG, Cader MZ, Poulin JF, Andres-Enguix I, Simoneau M, Gupta N, Boisvert K, Lafrenière F, McLaughlan S, Dubé MP et al.. (2010) A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat Med, 16 (10): 1157-60. [PMID:20871611]
69. Lazarenko RM, Willcox SC, Shu S, Berg AP, Jevtovic-Todorovic V, Talley EM, Chen X, Bayliss DA. (2010) Motoneuronal TASK channels contribute to immobilizing effects of inhalational general anesthetics. J Neurosci, 30 (22): 7691-704. [PMID:20519544]
70. Lengyel M, Czirják G, Enyedi P. (2016) Formation of Functional Heterodimers by TREK-1 and TREK-2 Two-pore Domain Potassium Channel Subunits. J Biol Chem, 291 (26): 13649-61. [PMID:27129242]
71. Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. (1996) TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J, 15 (5): 1004-11. [PMID:8605869]
72. Levitz J, Royal P, Comoglio Y, Wdziekonski B, Schaub S, Clemens DM, Isacoff EY, Sandoz G. (2016) Heterodimerization within the TREK channel subfamily produces a diverse family of highly regulated potassium channels. Proc Natl Acad Sci USA, 113 (15): 4194-9. [PMID:27035963]
73. Lloyd EE, Crossland RF, Phillips SC, Marrelli SP, Reddy AK, Taffet GE, Hartley CJ, Bryan Jr RM. (2011) Disruption of K(2P)6.1 produces vascular dysfunction and hypertension in mice. Hypertension, 58 (4): 672-8. [PMID:21876070]
74. Lolicato M, Riegelhaupt PM, Arrigoni C, Clark KA, Minor Jr DL. (2014) Transmembrane helix straightening and buckling underlies activation of mechanosensitive and thermosensitive K(2P) channels. Neuron, 84 (6): 1198-212. [PMID:25500157]
75. Lopes CM, Gallagher PG, Buck ME, Butler MH, Goldstein SA. (2000) Proton block and voltage gating are potassium-dependent in the cardiac leak channel Kcnk3. J Biol Chem, 275 (22): 16969-78. [PMID:10748056]
76. Lopes CM, Rohács T, Czirják G, Balla T, Enyedi P, Logothetis DE. (2005) PIP2 hydrolysis underlies agonist-induced inhibition and regulates voltage gating of two-pore domain K+ channels. J Physiol, 564 (Pt 1): 117-29. [PMID:15677683]
77. Lopes CM, Zilberberg N, Goldstein SA. (2001) Block of Kcnk3 by protons. Evidence that 2-P-domain potassium channel subunits function as homodimers. J Biol Chem, 276 (27): 24449-52. [PMID:11358956]
78. Lotshaw DP. (2007) Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem Biophys, 47 (2): 209-56. [PMID:17652773]
79. Ma L, Zhang X, Zhou M, Chen H. (2012) Acid-sensitive TWIK and TASK two-pore domain potassium channels change ion selectivity and become permeable to sodium in extracellular acidification. J Biol Chem, 287 (44): 37145-53. [PMID:22948150]
80. Maingret F, Fosset M, Lesage F, Lazdunski M, Honoré E. (1999) TRAAK is a mammalian neuronal mechano-gated K+ channel. J Biol Chem, 274 (3): 1381-7. [PMID:9880510]
81. Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. (1999) Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem, 274 (38): 26691-6. [PMID:10480871]
82. Martin S, Nishimune A, Mellor JR, Henley JM. (2007) SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature, 447 (7142): 321-5. [PMID:17486098]
83. McClenaghan C, Schewe M, Aryal P, Carpenter EP, Baukrowitz T, Tucker SJ. (2016) Polymodal activation of the TREK-2 K2P channel produces structurally distinct open states. J Gen Physiol, 147 (6): 497-505. [PMID:27241700]
84. Medhurst AD, Rennie G, Chapman CG, Meadows H, Duckworth MD, Kelsell RE, Gloger II, Pangalos MN. (2001) Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Res Mol Brain Res, 86 (1-2): 101-14. [PMID:11165377]
85. Millar JA, Barratt L, Southan AP, Page KM, Fyffe RE, Robertson B, Mathie A. (2000) A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci USA, 97 (7): 3614-8. [PMID:10725353]
86. Miller AN, Long SB. (2012) Crystal structure of the human two-pore domain potassium channel K2P1. Science, 335 (6067): 432-6. [PMID:22282804]
87. Mu D, Chen L, Zhang X, See LH, Koch CM, Yen C, Tong JJ, Spiegel L, Nguyen KC, Servoss A et al.. (2003) Genomic amplification and oncogenic properties of the KCNK9 potassium channel gene. Cancer Cell, 3 (3): 297-302. [PMID:12676587]
88. Nie X, Arrighi I, Kaissling B, Pfaff I, Mann J, Barhanin J, Vallon V. (2005) Expression and insights on function of potassium channel TWIK-1 in mouse kidney. Pflugers Arch, 451 (3): 479-88. [PMID:16025300]
89. Niemeyer MI, Cid LP, Barros LF, Sepúlveda FV. (2001) Modulation of the two-pore domain acid-sensitive K+ channel TASK-2 (KCNK5) by changes in cell volume. J Biol Chem, 276 (46): 43166-74. [PMID:11560934]
90. Niemeyer MI, Cid LP, González W, Sepúlveda FV. (2016) Gating, Regulation, and Structure in K2P K+ Channels: In Varietate Concordia?. Mol Pharmacol, 90 (3): 309-17. [PMID:27268784]
91. Niemeyer MI, González-Nilo FD, Zúñiga L, González W, Cid LP, Sepúlveda FV. (2007) Neutralization of a single arginine residue gates open a two-pore domain, alkali-activated K+ channel. Proc Natl Acad Sci U S A, 104 (2): 666-71. [PMID:17197424]
92. Noël J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A et al.. (2009) The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J, 28 (9): 1308-18. [PMID:19279663]
93. O'Kelly I. (2015) Endocytosis as a mode to regulate functional expression of two-pore domain potassium (K₂p) channels. Pflugers Arch, 467 (5): 1133-42. [PMID:25413469]
94. O'Kelly I, Butler MH, Zilberberg N, Goldstein SA. (2002) Forward transport. 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell, 111 (4): 577-88. [PMID:12437930]
95. O'Kelly I, Goldstein SA. (2008) Forward Transport of K2p3.1: mediation by 14-3-3 and COPI, modulation by p11. Traffic, 9 (1): 72-8. [PMID:17908283]
96. Patel AJ, Honoré E, Lesage F, Fink M, Romey G, Lazdunski M. (1999) Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci, 2 (5): 422-6. [PMID:10321245]
97. Pei L, Wiser O, Slavin A, Mu D, Powers S, Jan LY, Hoey T. (2003) Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function. Proc Natl Acad Sci USA, 100 (13): 7803-7. [PMID:12782791]
98. Pereira V, Busserolles J, Christin M, Devilliers M, Poupon L, Legha W, Alloui A, Aissouni Y, Bourinet E, Lesage F et al.. (2014) Role of the TREK2 potassium channel in cold and warm thermosensation and in pain perception. Pain, 155 (12): 2534-2544. [PMID:25239074]
99. Piechotta PL, Rapedius M, Stansfeld PJ, Bollepalli MK, Ehrlich G, Erhlich G, Andres-Enguix I, Fritzenschaft H, Decher N, Sansom MS et al.. (2011) The pore structure and gating mechanism of K2P channels. EMBO J, 30 (17): 3607-19. [PMID:21822218]
100. Plant LD & Goldstein SA. (2015) Two-Pore Domain Potassium Channels. In Handbook of Ion Channels Edited by Zheng J, Trudeau MC (CRC Press) 261-274. [ISBN:9781138198845]
101. Plant LD, Dementieva IS, Kollewe A, Olikara S, Marks JD, Goldstein SA. (2010) One SUMO is sufficient to silence the dimeric potassium channel K2P1. Proc Natl Acad Sci USA, 107 (23): 10743-8. [PMID:20498050]
102. Plant LD, Dowdell EJ, Dementieva IS, Marks JD, Goldstein SA. (2011) SUMO modification of cell surface Kv2.1 potassium channels regulates the activity of rat hippocampal neurons. J Gen Physiol, 137 (5): 441-54. [PMID:21518833]
103. Plant LD, Kemp PJ, Peers C, Henderson Z, Pearson HA. (2002) Hypoxic depolarization of cerebellar granule neurons by specific inhibition of TASK-1. Stroke, 33 (9): 2324-8. [PMID:12215606]
104. Plant LD, Rajan S, Goldstein SA. (2005) K2P channels and their protein partners. Curr Opin Neurobiol, 15 (3): 326-33. [PMID:15922586]
105. Plant LD, Zuniga L, Araki D, Marks JD, Goldstein SA. (2012) SUMOylation silences heterodimeric TASK potassium channels containing K2P1 subunits in cerebellar granule neurons. Sci Signal, 5 (251): ra84. [PMID:23169818]
106. Plant LD, Zúñiga L, Olikara S, Marks JD, Goldstein SAN. (2010) K2P1 Assembles with K2P3 or K2P9 to Form Sumo-Regulated Task Background Channels. Biophysical Journal, 98 (3 Suppl 1): 710a. DOI: 10.1016/j.bpj.2009.12.3894
107. Pountney DJ, Gulkarov I, Vega-Saenz de Miera E, Holmes D, Saganich M, Rudy B, Artman M, Coetzee WA. (1999) Identification and cloning of TWIK-originated similarity sequence (TOSS): a novel human 2-pore K+ channel principal subunit. FEBS Lett, 450 (3): 191-6. [PMID:10359073]
108. Rajan S, Plant LD, Rabin ML, Butler MH, Goldstein SA. (2005) Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell, 121 (1): 37-47. [PMID:15820677]
109. Rajan S, Wischmeyer E, Karschin C, Preisig-Müller R, Grzeschik KH, Daut J, Karschin A, Derst C. (2001) THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. J Biol Chem, 276 (10): 7302-11. [PMID:11060316]
110. Rajan S, Wischmeyer E, Xin Liu G, Preisig-Müller R, Daut J, Karschin A, Derst C. (2000) TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J Biol Chem, 275 (22): 16650-7. [PMID:10747866]
111. Rapedius M, Schmidt MR, Sharma C, Stansfeld PJ, Sansom MS, Baukrowitz T, Tucker SJ. (2012) State-independent intracellular access of quaternary ammonium blockers to the pore of TREK-1. Channels (Austin), 6 (6): 473-8. [PMID:22991046]
112. Reed AP, Bucci G, Abd-Wahab F, Tucker SJ. (2016) Dominant-Negative Effect of a Missense Variant in the TASK-2 (KCNK5) K+ Channel Associated with Balkan Endemic Nephropathy. PLoS One, 11 (5): e0156456. [PMID:27228168]
113. Salinas M, Reyes R, Lesage F, Fosset M, Heurteaux C, Romey G, Lazdunski M. (1999) Cloning of a new mouse two-P domain channel subunit and a human homologue with a unique pore structure. J Biol Chem, 274 (17): 11751-60. [PMID:10206991]
114. Sandoz G, Thümmler S, Duprat F, Feliciangeli S, Vinh J, Escoubas P, Guy N, Lazdunski M, Lesage F. (2006) AKAP150, a switch to convert mechano-, pH- and arachidonic acid-sensitive TREK K(+) channels into open leak channels. EMBO J, 25 (24): 5864-72. [PMID:17110924]
115. Sano Y, Inamura K, Miyake A, Mochizuki S, Kitada C, Yokoi H, Nozawa K, Okada H, Matsushime H, Furuichi K. (2003) A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J Biol Chem, 278 (30): 27406-12. [PMID:12754259]
116. Schewe M, Nematian-Ardestani E, Sun H, Musinszki M, Cordeiro S, Bucci G, de Groot BL, Tucker SJ, Rapedius M, Baukrowitz T. (2016) A Non-canonical Voltage-Sensing Mechanism Controls Gating in K2P K(+) Channels. Cell, 164 (5): 937-49. [PMID:26919430]
117. Schmidt C, Wiedmann F, Voigt N, Zhou XB, Heijman J, Lang S, Albert V, Kallenberger S, Ruhparwar A, Szabó G et al.. (2015) Upregulation of K(2P)3.1 K+ Current Causes Action Potential Shortening in Patients With Chronic Atrial Fibrillation. Circulation, 132 (2): 82-92. [PMID:25951834]
118. Simkin D, Cavanaugh EJ, Kim D. (2008) Control of the single channel conductance of K2P10.1 (TREK-2) by the amino-terminus: role of alternative translation initiation. J Physiol, 586 (23): 5651-63. [PMID:18845607]
119. Sirois JE, Lei Q, Talley EM, Lynch 3rd C, Bayliss DA. (2000) The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci, 20 (17): 6347-54. [PMID:10964940]
120. Streit AK, Netter MF, Kempf F, Walecki M, Rinné S, Bollepalli MK, Preisig-Müller R, Renigunta V, Daut J, Baukrowitz T et al.. (2011) A specific two-pore domain potassium channel blocker defines the structure of the TASK-1 open pore. J Biol Chem, 286 (16): 13977-84. [PMID:21362619]
121. Su Z, Brown EC, Wang W, MacKinnon R. (2016) Novel cell-free high-throughput screening method for pharmacological tools targeting K+ channels. Proc Natl Acad Sci U S A, 113 (20): 5748-53. [PMID:27091997]
122. Talley EM, Bayliss DA. (2002) Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. J Biol Chem, 277 (20): 17733-42. [PMID:11886861]
123. Talley EM, Lei Q, Sirois JE, Bayliss DA. (2000) TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron, 25 (2): 399-410. [PMID:10719894]
124. Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. (2001) Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci, 21 (19): 7491-505. [PMID:11567039]
125. Thomas D, Plant LD, Wilkens CM, McCrossan ZA, Goldstein SA. (2008) Alternative translation initiation in rat brain yields K2P2.1 potassium channels permeable to sodium. Neuron, 58 (6): 859-70. [PMID:18579077]
126. Toncheva D, Mihailova-Hristova M, Vazharova R, Staneva R, Karachanak S, Dimitrov P, Simeonov V, Ivanov S, Balabanski L, Serbezov D et al.. (2014) NGS nominated CELA1, HSPG2, and KCNK5 as candidate genes for predisposition to Balkan endemic nephropathy. Biomed Res Int, 2014: 920723. [PMID:24949484]
127. Tulleuda A, Cokic B, Callejo G, Saiani B, Serra J, Gasull X. (2011) TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury. Mol Pain, 7: 30. [PMID:21527011]
128. Veale EL, Al-Moubarak E, Bajaria N, Omoto K, Cao L, Tucker SJ, Stevens EB, Mathie A. (2014) Influence of the N terminus on the biophysical properties and pharmacology of TREK1 potassium channels. Mol Pharmacol, 85 (5): 671-81. [PMID:24509840]
129. Veale EL, Mathie A. (2016) Aristolochic acid, a plant extract used in the treatment of pain and linked to Balkan endemic nephropathy, is a regulator of K2P channels. Br J Pharmacol, 173 (10): 1639-52. [PMID:26914156]
130. Vivier D, Bennis K, Lesage F, Ducki S. (2016) Perspectives on the Two-Pore Domain Potassium Channel TREK-1 (TWIK-Related K(+) Channel 1). A Novel Therapeutic Target?. J Med Chem, 59 (11): 5149-57. [PMID:26588045]
131. Wang S, Benamer N, Zanella S, Kumar NN, Shi Y, Bévengut M, Penton D, Guyenet PG, Lesage F, Gestreau C et al.. (2013) TASK-2 channels contribute to pH sensitivity of retrotrapezoid nucleus chemoreceptor neurons. J Neurosci, 33 (41): 16033-44. [PMID:24107938]
132. Wang W, Kiyoshi CM, Du Y, Ma B, Alford CC, Chen H, Zhou M. (2016) mGluR3 Activation Recruits Cytoplasmic TWIK-1 Channels to Membrane that Enhances Ammonium Uptake in Hippocampal Astrocytes. Mol Neurobiol, 53 (9): 6169-6182. [PMID:26553349]
133. Wang W, Putra A, Schools GP, Ma B, Chen H, Kaczmarek LK, Barhanin J, Lesage F, Zhou M. (2013) The contribution of TWIK-1 channels to astrocyte K(+) current is limited by retention in intracellular compartments. Front Cell Neurosci, 7: 246. [PMID:24368895]
134. Warth R, Barrière H, Meneton P, Bloch M, Thomas J, Tauc M, Heitzmann D, Romeo E, Verrey F, Mengual R et al.. (2004) Proximal renal tubular acidosis in TASK2 K+ channel-deficient mice reveals a mechanism for stabilizing bicarbonate transport. Proc Natl Acad Sci USA, 101 (21): 8215-20. [PMID:15141089]
135. Yamamoto Y, Hatakeyama T, Taniguchi K. (2009) Immunohistochemical colocalization of TREK-1, TREK-2 and TRAAK with TRP channels in the trigeminal ganglion cells. Neurosci Lett, 454 (2): 129-33. [PMID:19429069]
136. Yellen G. (1998) The moving parts of voltage-gated ion channels. Q Rev Biophys, 31 (3): 239-95. [PMID:10384687]
137. Zilberberg N, Ilan N, Goldstein SA. (2001) KCNKØ: opening and closing the 2-P-domain potassium leak channel entails "C-type" gating of the outer pore. Neuron, 32 (4): 635-48. [PMID:11719204]
138. Zilberberg N, Ilan N, Gonzalez-Colaso R, Goldstein SA. (2000) Opening and closing of KCNKO potassium leak channels is tightly regulated. J Gen Physiol, 116 (5): 721-34. [PMID:11055999]
139. Zuzarte M, Heusser K, Renigunta V, Schlichthörl G, Rinné S, Wischmeyer E, Daut J, Schwappach B, Preisig-Müller R. (2009) Intracellular traffic of the K+ channels TASK-1 and TASK-3: role of N- and C-terminal sorting signals and interaction with 14-3-3 proteins. J Physiol, 587 (Pt 5): 929-52. [PMID:19139046]