GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Cyclic nucleotide-regulated channels (CNG): Introduction
Introduction
Cyclic nucleotide-regulated channels are members of the voltage-gated cation channel superfamily. There are two subgroups of cyclic nucleotide-regulated channels: the cyclic nucleotide-gated (CNG) channels and the hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels. CNG channels play a key role in sensory transduction in retinal photoreceptors and olfactory neurons where they transduce stimulus-mediated changes in the concentration of the second messengers cGMP and cAMP into changes of the membrane potential. HCN channels are expressed throughout the nervous system and play key roles in controlling excitability and other electrical properties (e.g. firing pattern) of neurons. Moreover, HCN channels are involved in cardiac pacemaking and ventricular repolarization. This article presents a brief introduction to the structural, functional and pharmacological properties of cyclic nucleotide-regulated channels and gives an overview on their role in physiology and disease.
Cyclic Nucleotide-Gated Channels
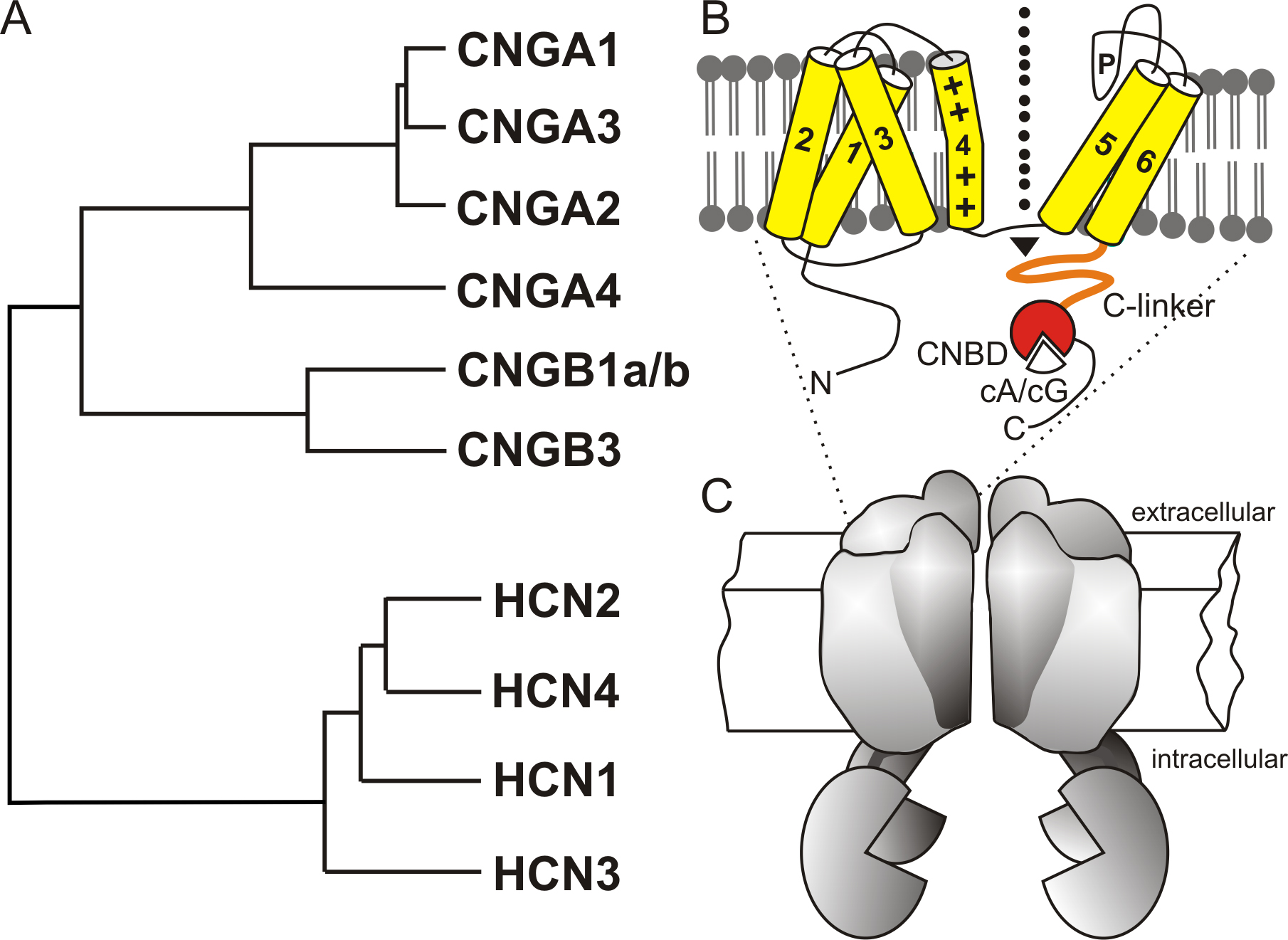
CNG cation channels are ion channels whose activation is mediated by the binding of cGMP or cAMP to the channel protein [3,34,43]. CNG channels are expressed in the cilia of olfactory neurones and in outer segments of rod and cone photoreceptor neurones, where they play key roles in sensory transduction. CNG channels are heterotetramers composed of homologous A subunits (CNGA1-CNGA4) and B subunits (CNGB1 and CNGB3) [8] (Fig. 1). There are two splice variants of CNGB1 (CNGB1a and CNGB1b) that differ from each with respect to the cytosolic N-terminus [12,36,59]. Like other members of the voltage-gated cation channel family CNG channel A and B subunits contain six transmembrane α helices (S1-S6) including a pore loop between S5 and S6 that forms the ion selectivity filter. In the cytosolic C terminus, CNG channel subunits carry a cyclic nucleotide-binding domain (CNBD) that is connected with the transmembrane core via the C-linker, an α-helix folded domain of about 90 amino acids. CNBD and C-linker serve as cyclic nucleotide sensor and principal activation domain of the channel. Although CNG channel subunits contain a positively charged S4 segment the channels are not gated by voltage. The CNBD of CNG channels reveals significant sequence similarity to the CNBDs of other cyclic nucleotide receptors [32]. Like other members of voltage-gated cation channel superfamily, CNG channel subunits assemble to tetrameric complex. The subunit stoichiometries have been determined for the channels expressed in rod photoreceptors (3 CNGA1: 1 CNGB1a) [69,72,74], and olfactory neurons (2 CNGA2: 1 CNGA4: 1 CNGB1b) [73]. The cone CNG channel is also thought to have a 3:1 stoichiometry (3 CNGA3: 1 CNGB3) ([60] but [51]). The specific functions of CNGA2-4, CNGB1 and CNGB3 subunits have been elucidated by gene deletion in mice [4,9,16,29,46].
CNG channels pass monovalent cations, such as Na+ and K+, but do not discriminate between them. Calcium is also permeable but at the same time acts as a voltage-dependent blocker of monovalent cation permeability [18,23]. Moreover, Ca2+ provides feedback inhibition of CNG channel activity by binding to calmodulin [34,43]. CNG channels reveal a higher sensitivity for cGMP than for cAMP. The extent of ligand discrimination varies significantly between the individual CNG channel types. Photoreceptor channels strongly discriminate between cGMP and cAMP, whereas the olfactory channel is almost equally sensitive to both ligands.
Figure 1. A, phylogenetic tree of mammalian cyclic nucleotide-regulated cation channels. B, domain structure of CNG and HCN channel subunits. 1-6, transmembrane helices; P, pore-loop harboring the ion selectivity filter; C-linker (C-terminal linker domain); CNBD, cyclic nucleotide binding domain. The dotted arrow indicates the flow of cations through the pore. C, channel tetramer.
CNG Channelopathies
Impaired function of photoreceptor CNG channel subunits has been implicated in the pathologies of two human retinal diseases, retinitis pigmentosa (RP) and achromatopsia. RP comprise a genetically diverse group of degenerative diseases affecting retinal photoreceptors [25]. The most common symptoms of RP include night blindness, progressive concentric reduction of the visual field, and abnormal accumulation of pigmentation in the retina [31]. In most cases, RP finally leads to legal blindness. At the cellular level, RP is characterized by a primary impairment or total loss of rod function followed by secondary degeneration of cones ("bystander effect"). Loss of function mutations in CNGA1 account for about 1% of cases of autosomal recessive RP (arRP) [17,25]. Mutations in the CNGB1a subunit of the rod CNG channel were also identified in RP patients and account for ~4% of arRP cases [2,61].
The functional loss of either one of the two cone photoreceptor CNG channel subunits (CNGA3 [35] and CNGB3 [64]) causes achromatopsia (ACHM). ACHM is characterized by a total loss of cone function resulting in loss of color discrimination, photophobia, nystagmus, very poor visual acuity and foveomacular degeneration. Together, mutation in CNGA3 and CNGB3 account for about 80% of ACHM cases.
So far, mutations in the olfactory CNG channel complex have not been associated with a human disease.
Drugs That Act on CNG Channels
Several drugs have been reported to block CNG channels, although not with very high affinity. L-cis-diltiazem has been studied most extensively. It blocks CNG channels in a voltage-dependent manner at micromolar concentration [26]. The D-cis-enantiomer of diltiazem that is used therapeutically as a blocker of the L-type calcium channel is much less effective than the L-cis-enantiomer. High-affinity binding of L-cis-diltiazem is only seen in heteromeric CNG channels containing the CNGB1 subunit [12]. CNG channels are also moderately sensitive to block by some other inhibitors of the L-type calcium channel (e.g. nifedipine), the local anesthetic tetracaine, and calmodulin antagonists [34]. Interestingly, LY83583 blocks both the soluble guanylate cyclase and some CNG channels at similar concentrations [37]. H-8, which has been widely used as a nonspecific cyclic nucleotide-dependent protein kinase inhibitor, blocks CNG channels, although at significantly higher concentrations than those needed to inhibit protein kinases [68].
Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels
HCN and CNG channels share the same principal domain structure that is composed of six transmembrane segments, ion-conducting pore-loop, C-linker and CNBD [5,33,54] (Fig. 1). In contrast to CNG channels that are not operated by voltage, HCN channels require a change in the membrane potential to be activated. However, in contrast to most other voltage-gated channels, HCN channels open upon membrane hyperpolarization and close at positive potential. The cyclic nucleotides cAMP and cGMP enhance HCN channel activity by shifting the activation curve of the channels to more positive voltages. The stimulatory effect of cyclic nucleotides does not depend on protein phosphorylation but is caused by direct interaction with the CNBD of the HCN channel protein. The current produced by HCN channels, termed Ih, If, or Iq, is found in a variety of excitable cells, including neurones, cardiac pacemaker cells, and photoreceptors [5,50,54]. The best-understood function of Ih is to control heart rate and rhythm by acting as "pacemaker current" in the sinoatrial (SA) node [1,66]. Ih is activated during the membrane hyperpolarization following the termination of an action potential and provides an inward Na+ current that slowly depolarizes the plasma membrane. Sympathetic stimulation of SA node cells raises cAMP levels and increases Ih, thus accelerating diastolic depolarization and heart rate. Stimulation of muscarinic acetylcholine receptors slows down heart rate by the opposite action. Ih was also found to play a role in ventricular repolarization [20]. In neurons, Ih fulfills diverse functions, including generation of pacemaker potentials ("neuronal pacemaking"), determination of resting potential, dendritic integration, control of synaptic transmission and plasticity [5,54].
In mammals, the HCN channel family comprises four members (HCN1-HCN4) that share approximately 60% sequence identity to each other [24,41-42,55]. HCN channels contain six-transmembrane helices (S1-S6) and assemble in tetramers [71]. There is evidence that HCN subunits can coassemble to form heterotetramers [45]. The S4 segment of the channels is positively charged and serves as voltage sensor [47]. The C terminus of HCN channels contains a CNBD that together with the C-linker confers regulation by cyclic nucleotides [67,71]. While a high resolution structure of a complete HCN channel has not yet been published, the crystal structure of the CNBD including C-linker has been determined for several HCN channel types [22,39,70-71]. Collectively, these studies indicate that the C-linker-CNBD domain assembles as a fourfold symmetric tetramer in which the primary subunit interactions are provided by the C-linkers. Binding of cAMP to the CNBD induces a rearrangement of the C-linkers that stabilizes the open channel conformation. Functional properties of HCN channels as well as cell surface expression may also be modulated by accessory proteins that are forming complexes with the channel [5,38]. The best characterized HCN channel interacting protein is the tetratricopeptide repeat-containing Rab8b interacting protein(TRIP8b) that in neurons forms tight complexes with the CNBD of HCN channels [56,75]. TRIP8b inhibits HCN channel gating by both restricting cAMP binding and interfering with the conformational transition necessary to couple cAMP binding to channel activation [13,57].
When expressed in heterologous systems, all four HCN channels generate currents displaying the typical features of native Ih: 1) activation by membrane hyperpolarisation; 2) permeation of Na+ and K+ with a permeability ratio PNa/PK of approximately 0.2; 3) positive shift of voltage-dependence of channel activation by direct binding of cyclic nucleotides; and 4) channel block by extracellular Cs+. The HCN1-HCN4 currents mainly differ from each other with regard to their speed of activation and the extent by which they are modulated by cyclic nucleotides. HCN1 is the fastest channel, followed by HCN2, HCN3, and HCN4. Unlike HCN2 and HCN4, whose activation curves are profoundly shifted by cyclic nucleotides, HCN1 and HCN3 are only weakly affected by these cyclic nucleotides [5,54].
HCN channels are found in neurons and heart cells. In SA node cells of vertebrates, HCN4 and HCN1 represent the predominantly expressed HCN channel isoforms that underlie 70% (HCN4) and 30% (HCN1) of total Ih, respectively [21,30,30,62]. HCN2 is also expressed in SA node but seems to play only a minor role in cardiac pacemaking [28,40]. In brain, all four HCN subunits have been detected [49]. The expression levels and regional distribution of the four HCN channel types vary profoundly between the respective channel types. HCN2 is the most abundant neuronal channel and is found almost ubiquitously in the brain. By contrast, HCN1 and HCN4 are enriched in specific regions of the brain such as thalamus (HCN4) or hippocampus (HCN1). HCN3 is expressed at low density in most parts of the brain but is enriched in olfactory bulb and some hypothalamic nuclei [11]. HCN channels have also been detected in the retina [48] and some peripheral neurones such as dorsal root ganglion neurones [19,44]. The specific roles of individual HCN channel types have been defined by analysis of HCN channel-deficient mouse lines [5,10,27].
HCN Channelopathies
More than 20 mutations in the HCN4 gene have been associated with sinus node dysfunction [65]. Clinical symptoms associated with these mutations include sinus bradycardia and in some cases also more severe arrhythmic conditions including QT prolongation, tachycardia-bradycardia syndrome, AV block and atrial fibrillation. Clincical symptoms not related to cardiac dysfunction have not been reported for carriers of HCN4 mutations. More recently, mutations of HCN1 and HCN2 have been associated with human epilepsy [15]. Studies in heterologous expression systems revealed that these mutations had qualitatively diverse effects on HCN channel currents ranging from loss of function to gain of function [15]. So far, channelopathies have not been reported for HCN3.
Drugs That Act on HCN Channels
HCN channels have been considered as potential therapeutic target for a variety of diseases [52,58]. Given the key role of HCN channels in cardiac pacemaking, these channels are promising pharmacological targets for the development of drugs used in the treatment of cardiac arrhythmias and ischemic heart disease. Several blockers of native Ih channels are known. The most extensively studied blocker is ZD7288 [7]. Low micromolar concentrations of this agent specifically block both native Ih and cloned HCN channels in a voltage-dependent manner. Three other use-dependent blockers of Ih are ivabradine [6], zatebradine [53], and cilobradine [63]. Structurally, these substances are related to verapamil, a classic L-type calcium channel blocker. These agents block Ih at concentrations comparable to ZD7288. Ivabradine has been approved as a heart rate-lowering agent in the therapy of angina pectoris. The way for more specific HCN isoform blockers was paved with the synthesis of ivabrandine derivatives. The testing of selected compounds on native guinea pig sinoatrial node cells and neurons from mouse dorsal root ganglion (DRG) by patch-clamp recordings revealed E18 as a selective blocker for hHCN4 and MEL57A-specific blockage of mHCN1 [14]. Compounds targeting specific HCN channels may also offer excellent opportunities for the development of novel anticonvulsant, anesthetic, analgesic and antipsychotic drugs.
References
1. Barbuti A, DiFrancesco D. (2008) Control of cardiac rate by "funny" channels in health and disease. Ann N Y Acad Sci, 1123: 213-23. [PMID:18375593]
2. Bareil C, Hamel CP, Delague V, Arnaud B, Demaille J, Claustres M. (2001) Segregation of a mutation in CNGB1 encoding the beta-subunit of the rod cGMP-gated channel in a family with autosomal recessive retinitis pigmentosa. Hum Genet, 108 (4): 328-34. [PMID:11379879]
3. Biel M, Michalakis S. (2009) Cyclic nucleotide-gated channels. In Handbook of Experimental Pharmacology Edited by Schmidt HH, Hofmann F, Stasch JP (Springer) 111-136. [ISBN:9783540689607]
4. Biel M, Seeliger M, Pfeifer A, Kohler K, Gerstner A, Ludwig A, Jaissle G, Fauser S, Zrenner E, Hofmann F. (1999) Selective loss of cone function in mice lacking the cyclic nucleotide-gated channel CNG3. Proc Natl Acad Sci USA, 96 (13): 7553-7. [PMID:10377453]
5. Biel M, Wahl-Schott C, Michalakis S, Zong X. (2009) Hyperpolarization-activated cation channels: from genes to function. Physiol Rev, 89 (3): 847-85. [PMID:19584315]
6. Bois P, Bescond J, Renaudon B, Lenfant J. (1996) Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol, 118 (4): 1051-7. [PMID:8799581]
7. BoSmith RE, Briggs I, Sturgess NC. (1993) Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br J Pharmacol, 110 (1): 343-9. [PMID:7693281]
8. Bradley J, Frings S, Yau KW, Reed R. (2001) Nomenclature for ion channel subunits. Science, 294 (5549): 2095-6. [PMID:11764791]
9. Brunet LJ, Gold GH, Ngai J. (1996) General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron, 17 (4): 681-93. [PMID:8893025]
10. Bucchi A, Barbuti A, Difrancesco D, Baruscotti M. (2012) Funny Current and Cardiac Rhythm: Insights from HCN Knockout and Transgenic Mouse Models. Front Physiol, 3: 240. [PMID:22783204]
11. Cao-Ehlker X, Zong X, Hammelmann V, Gruner C, Fenske S, Michalakis S, Wahl-Schott C, Biel M. (2013) Up-regulation of hyperpolarization-activated cyclic nucleotide-gated channel 3 (HCN3) by specific interaction with K+ channel tetramerization domain-containing protein 3 (KCTD3). J Biol Chem, 288 (11): 7580-9. [PMID:23382386]
12. Chen TY, Peng YW, Dhallan RS, Ahamed B, Reed RR, Yau KW. (1993) A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature, 362 (6422): 764-7. [PMID:7682292]
13. DeBerg HA, Bankston JR, Rosenbaum JC, Brzovic PS, Zagotta WN, Stoll S. (2015) Structural mechanism for the regulation of HCN ion channels by the accessory protein TRIP8b. Structure, 23 (4): 734-44. [PMID:25800552]
14. Del Lungo M, Melchiorre M, Guandalini L, Sartiani L, Mugelli A, Koncz I, Szel T, Varro A, Romanelli MN, Cerbai E. (2012) Novel blockers of hyperpolarization-activated current with isoform selectivity in recombinant cells and native tissue. Br J Pharmacol, 166 (2): 602-16. [PMID:22091830]
15. DiFrancesco JC, DiFrancesco D. (2015) Dysfunctional HCN ion channels in neurological diseases. Front Cell Neurosci, 6: 174. [PMID:25805968]
16. Ding XQ, Harry CS, Umino Y, Matveev AV, Fliesler SJ, Barlow RB. (2009) Impaired cone function and cone degeneration resulting from CNGB3 deficiency: down-regulation of CNGA3 biosynthesis as a potential mechanism. Hum Mol Genet, 18 (24): 4770-80. [PMID:19767295]
17. Dryja TP, Finn JT, Peng YW, McGee TL, Berson EL, Yau KW. (1995) Mutations in the gene encoding the alpha subunit of the rod cGMP-gated channel in autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci USA, 92 (22): 10177-81. [PMID:7479749]
18. Dzeja C, Hagen V, Kaupp UB, Frings S. (1999) Ca2+ permeation in cyclic nucleotide-gated channels. EMBO J, 18 (1): 131-44. [PMID:9878057]
19. Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. (2011) HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science, 333 (6048): 1462-6. [PMID:21903816]
20. Fenske S, Krause S, Biel M, Wahl-Schott C. (2011) The role of HCN channels in ventricular repolarization. Trends Cardiovasc Med, 21 (8): 216-20. [PMID:22902068]
21. Fenske S, Krause SC, Hassan SI, Becirovic E, Auer F, Bernard R, Kupatt C, Lange P, Ziegler T, Wotjak CT et al.. (2013) Sick sinus syndrome in HCN1-deficient mice. Circulation, 128 (24): 2585-94. [PMID:24218458]
22. Flynn GE, Black KD, Islas LD, Sankaran B, Zagotta WN. (2007) Structure and rearrangements in the carboxy-terminal region of SpIH channels. Structure, 15 (6): 671-82. [PMID:17562314]
23. Frings S, Seifert R, Godde M, Kaupp UB. (1995) Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron, 15 (1): 169-79. [PMID:7542461]
24. Gauss R, Seifert R, Kaupp UB. (1998) Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature, 393 (6685): 583-7. [PMID:9634235]
25. Hartong DT, Berson EL, Dryja TP. (2006) Retinitis pigmentosa. Lancet, 368 (9549): 1795-809. [PMID:17113430]
26. Haynes LW. (1992) Block of the cyclic GMP-gated channel of vertebrate rod and cone photoreceptors by l-cis-diltiazem. J Gen Physiol, 100 (5): 783-801. [PMID:1282145]
27. Herrmann S, Hofmann F, Stieber J, Ludwig A. (2012) HCN channels in the heart: lessons from mouse mutants. Br J Pharmacol, 166 (2): 501-9. [PMID:22141457]
28. Herrmann S, Layh B, Ludwig A. (2011) Novel insights into the distribution of cardiac HCN channels: an expression study in the mouse heart. J Mol Cell Cardiol, 51 (6): 997-1006. [PMID:21945247]
29. Hüttl S, Michalakis S, Seeliger M, Luo DG, Acar N, Geiger H, Hudl K, Mader R, Haverkamp S, Moser M et al.. (2005) Impaired channel targeting and retinal degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGB1. J Neurosci, 25 (1): 130-8. [PMID:15634774]
30. Ishii TM, Takano M, Xie LH, Noma A, Ohmori H. (1999) Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node. J Biol Chem, 274 (18): 12835-9. [PMID:10212270]
31. Kalloniatis M, Fletcher EL. (2004) Retinitis pigmentosa: understanding the clinical presentation, mechanisms and treatment options. Clin Exp Optom, 87 (2): 65-80. [PMID:15040773]
32. Kaupp UB, Niidome T, Tanabe T, Terada S, Bönigk W, Stühmer W, Cook NJ, Kangawa K, Matsuo H, Hirose T. (1989) Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature, 342 (6251): 762-6. [PMID:2481236]
33. Kaupp UB, Seifert R. (2001) Molecular diversity of pacemaker ion channels. Annu Rev Physiol, 63: 235-57. [PMID:11181956]
34. Kaupp UB, Seifert R. (2002) Cyclic nucleotide-gated ion channels. Physiol Rev, 82 (3): 769-824. [PMID:12087135]
35. Kohl S, Marx T, Giddings I, Jägle H, Jacobson SG, Apfelstedt-Sylla E, Zrenner E, Sharpe LT, Wissinger B. (1998) Total colourblindness is caused by mutations in the gene encoding the alpha-subunit of the cone photoreceptor cGMP-gated cation channel. Nat Genet, 19 (3): 257-9. [PMID:9662398]
36. Körschen HG, Illing M, Seifert R, Sesti F, Williams A, Gotzes S, Colville C, Müller F, Dosé A, Godde M et al.. (1995) A 240 kDa protein represents the complete beta subunit of the cyclic nucleotide-gated channel from rod photoreceptor. Neuron, 15 (3): 627-36. [PMID:7546742]
37. Leinders-Zufall T, Zufall F. (1995) Block of cyclic nucleotide-gated channels in salamander olfactory receptor neurons by the guanylyl cyclase inhibitor LY83583. J Neurophysiol, 74 (6): 2759-62. [PMID:8747232]
38. Lewis AS, Estep CM, Chetkovich DM. (2010) The fast and slow ups and downs of HCN channel regulation. Channels (Austin), 4 (3): 215-31. [PMID:20305382]
39. Lolicato M, Nardini M, Gazzarrini S, Möller S, Bertinetti D, Herberg FW, Bolognesi M, Martin H, Fasolini M, Bertrand JA et al.. (2011) Tetramerization dynamics of C-terminal domain underlies isoform-specific cAMP gating in hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem, 286 (52): 44811-20. [PMID:22006928]
40. Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F. (2003) Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J, 22 (2): 216-24. [PMID:12514127]
41. Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. (1998) A family of hyperpolarization-activated mammalian cation channels. Nature, 393 (6685): 587-91. [PMID:9634236]
42. Ludwig A, Zong X, Stieber J, Hullin R, Hofmann F, Biel M. (1999) Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO J, 18 (9): 2323-9. [PMID:10228147]
43. Matulef K, Zagotta WN. (2003) Cyclic nucleotide-gated ion channels. Annu Rev Cell Dev Biol, 19: 23-44. [PMID:14570562]
44. Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A. (2001) Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem, 268 (6): 1646-52. [PMID:11248683]
45. Much B, Wahl-Schott C, Zong X, Schneider A, Baumann L, Moosmang S, Ludwig A, Biel M. (2003) Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem, 278 (44): 43781-6. [PMID:12928435]
46. Munger SD, Lane AP, Zhong H, Leinders-Zufall T, Yau KW, Zufall F, Reed RR. (2001) Central role of the CNGA4 channel subunit in Ca2+-calmodulin-dependent odor adaptation. Science, 294 (5549): 2172-5. [PMID:11739959]
47. Männikkö R, Elinder F, Larsson HP. (2002) Voltage-sensing mechanism is conserved among ion channels gated by opposite voltages. Nature, 419 (6909): 837-41. [PMID:12397358]
48. Müller F, Scholten A, Ivanova E, Haverkamp S, Kremmer E, Kaupp UB. (2003) HCN channels are expressed differentially in retinal bipolar cells and concentrated at synaptic terminals. Eur J Neurosci, 17 (10): 2084-96. [PMID:12786975]
49. Notomi T, Shigemoto R. (2004) Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol, 471 (3): 241-76. [PMID:14991560]
50. Pape HC. (1996) Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol, 58: 299-327. [PMID:8815797]
51. Peng C, Rich ED, Varnum MD. (2004) Subunit configuration of heteromeric cone cyclic nucleotide-gated channels. Neuron, 42 (3): 401-10. [PMID:15134637]
52. Postea O, Biel M. (2011) Exploring HCN channels as novel drug targets. Nat Rev Drug Discov, 10 (12): 903-14. [PMID:22094868]
53. Raes A, Van de Vijver G, Goethals M, van Bogaert PP. (1998) Use-dependent block of Ih in mouse dorsal root ganglion neurons by sinus node inhibitors. Br J Pharmacol, 125 (4): 741-50. [PMID:9831910]
54. Robinson RB, Siegelbaum SA. (2003) Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol, 65: 453-80. [PMID:12471170]
55. Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. (1998) Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell, 93 (5): 717-29. [PMID:9630217]
56. Santoro B, Wainger BJ, Siegelbaum SA. (2004) Regulation of HCN channel surface expression by a novel C-terminal protein-protein interaction. J Neurosci, 24 (47): 10750-62. [PMID:15564593]
57. Saponaro A, Pauleta SR, Cantini F, Matzapetakis M, Hammann C, Donadoni C, Hu L, Thiel G, Banci L, Santoro B et al.. (2014) Structural basis for the mutual antagonism of cAMP and TRIP8b in regulating HCN channel function. Proc Natl Acad Sci U S A, 111 (40): 14577-82. [PMID:25197093]
58. Sartiani L, Romanelli MN, Mugelli A, Cerbai E. (2015) Updates on HCN Channels in the Heart: Function, Dysfunction and Pharmacology. Curr Drug Targets, 16 (8): 868-76. [PMID:26028050]
59. Sautter A, Zong X, Hofmann F, Biel M. (1998) An isoform of the rod photoreceptor cyclic nucleotide-gated channel beta subunit expressed in olfactory neurons. Proc Natl Acad Sci USA, 95 (8): 4696-701. [PMID:9539801]
60. Shuart NG, Haitin Y, Camp SS, Black KD, Zagotta WN. (2011) Molecular mechanism for 3:1 subunit stoichiometry of rod cyclic nucleotide-gated ion channels. Nat Commun, 2: 457. [PMID:21878911]
61. Simpson DA, Clark GR, Alexander S, Silvestri G, Willoughby CE. (2011) Molecular diagnosis for heterogeneous genetic diseases with targeted high-throughput DNA sequencing applied to retinitis pigmentosa. J Med Genet, 48 (3): 145-51. [PMID:21147909]
62. Stieber J, Herrmann S, Feil S, Löster J, Feil R, Biel M, Hofmann F, Ludwig A. (2003) The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci USA, 100 (25): 15235-40. [PMID:14657344]
63. Stieber J, Wieland K, Stöckl G, Ludwig A, Hofmann F. (2006) Bradycardic and proarrhythmic properties of sinus node inhibitors. Mol Pharmacol, 69 (4): 1328-37. [PMID:16387796]
64. Sundin OH, Yang JM, Li Y, Zhu D, Hurd JN, Mitchell TN, Silva ED, Maumenee IH. (2000) Genetic basis of total colourblindness among the Pingelapese islanders. Nat Genet, 25 (3): 289-93. [PMID:10888875]
65. Verkerk AO, Wilders R. (2015) Pacemaker activity of the human sinoatrial node: an update on the effects of mutations in HCN4 on the hyperpolarization-activated current. Int J Mol Sci, 16 (2): 3071-94. [PMID:25642760]
66. Wahl-Schott C, Fenske S, Biel M. (2014) HCN channels: new roles in sinoatrial node function. Curr Opin Pharmacol, 15: 83-90. [PMID:24441197]
67. Wainger BJ, DeGennaro M, Santoro B, Siegelbaum SA, Tibbs GR. (2001) Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature, 411 (6839): 805-10. [PMID:11459060]
68. Wei JY, Cohen ED, Barnstable CJ. (1997) Direct blockade of both cloned rat rod photoreceptor cyclic nucleotide-gated non-selective cation (CNG) channel alpha-subunit and native CNG channels from Xenopus rod outer segments by H-8, a non-specific cyclic nucleotide-dependent protein kinase inhibitor. Neurosci Lett, 233 (1): 37-40. [PMID:9324234]
69. Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB. (2002) Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron, 36 (5): 881-9. [PMID:12467591]
70. Xu X, Vysotskaya ZV, Liu Q, Zhou L. (2010) Structural basis for the cAMP-dependent gating in the human HCN4 channel. J Biol Chem, 285 (47): 37082-91. [PMID:20829353]
71. Zagotta WN, Olivier NB, Black KD, Young EC, Olson R, Gouaux E. (2003) Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature, 425 (6954): 200-5. [PMID:12968185]
72. Zheng J, Trudeau MC, Zagotta WN. (2002) Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron, 36 (5): 891-6. [PMID:12467592]
73. Zheng J, Zagotta WN. (2004) Stoichiometry and assembly of olfactory cyclic nucleotide-gated channels. Neuron, 42 (3): 411-21. [PMID:15134638]
74. Zhong H, Molday LL, Molday RS, Yau KW. (2002) The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature, 420 (6912): 193-8. [PMID:12432397]
75. Zolles G, Wenzel D, Bildl W, Schulte U, Hofmann A, Müller CS, Thumfart JO, Vlachos A, Deller T, Pfeifer A et al.. (2009) Association with the auxiliary subunit PEX5R/Trip8b controls responsiveness of HCN channels to cAMP and adrenergic stimulation. Neuron, 62 (6): 814-25. [PMID:19555650]