
GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Angiotensin receptors: Introduction
General
The classification of the angiotensin receptors is based on the sequence of genes, structural features of the encoded proteins and their pharmacological properties (i.e. ligand-binding affinities and functional efficacies for selective agonists and antagonists), including the signal transduction mechanisms. Two types of mammalian receptors for the major renin-angiotensin receptor hormone angiotensinII (Ang II) have been cloned [28,43,54-55] and are designated by the abbreviations AT1 for angiotensin type 1 receptor and AT2 for angiotensin type 2 receptor [8,15]. The human genome contains single genes for AT1 and AT2. In rodents and zebra fish two genes encoding subtypes AT1a and AT1b exist [22,41,70]. Previous suggestion for a distinct Ang III(angiotensin 2-8) receptor, i.e. AT3 [40] or MAS [35] receptor has disappeared. The AT2 receptor mediated effects in various tissues indeed may critically require local conversion of Ang II to Ang III through the involvement of resident aminopeptidase in specific tissues such as kidney and brain [30,45,60]. The mammalian receptor for the Ang IV(angiotensin 3-8), named AT4 has been cloned and broadly characterized [2,10,34,37]. The AT4 receptor is insulin-regulated membrane aminopeptidase and a selective receptor for Ang IV. In addition, the receptor postulated for angiotensin 1-7 [42], has been tentatively assigned as the GPCR encoded by MAS oncogene [64]. The existence of other receptor types, which are neither AT1 nor AT2, has been proposed on pharmacological grounds but has not been unequivocally proven. In addition, a cytosolic binding protein and a nuclear receptor have been reported but are still poorly characterized [74,77]. A membrane-bound metalloendopeptidase, neurolysin (EC 3.4.24.16) was identified as the novel non-AT1, non-AT2 Ang II binding protein in rodent and human brain membranes [75,83].
Most of the conventional actions of Ang II, such as maintainance of blood pressure and glomerular filtration rate in response to extracellular volume depletion are mediated through the AT1receptor. Consequently, significantly greater advances have been made in developing pharmacological agents targeting the AT1 receptor compared to the others. Although Ang II has been viewed as a blood-borne hormone produced in the circulation, it is now established that in many tissues such as the brain, kidney, heart, and blood vessels, Ang II is formed locally and functions as a paracrine and autocrine hormone [31].
Pharmacological characteristics
The AT1 and AT2 receptors are membrane glycoproteins, members of the seven transmembrane-domain (7TM), G protein-coupled receptor superfamily [16,31-32]. Pharmacologically, the typical features of AT1 receptors are their selective affinity for biphenylimidazoles (typified by losartan) and their insensitivity to tetrahydroimidazopyridines (such as PD123177). The AT1 receptor is inactivated upon treatment with thiol reagents. By contrast, the AT2 receptor has low affinity for losartan and high affinity for PD123177 and a peptide derivative, CGP42112 (Figures 1, 2). The AT2 receptor is activated when treated with thiol reagents [24]. Although the sequence homology between the AT1 and AT2 receptors is only 30%, the natural ligand angiotensin II and peptide antagonists such as [Sar1, Ile8] Ang II do not distinguish between the two receptors. The MAS receptor is not pharmacologically related to AT1 and AT2 receptors.
The AT1 Receptor
In humans, the gene encoding the AT1 receptor is located on chromosome 3. The AT1A and AT1B originate from two closely related, but distinct genes are located on chromosomes 17 and 2, respectively in rodents. AT1A and AT1B receptors differ in 19 amino acids, mainly in the C-terminal region. The rodent AT1A and AT1B receptors are pharmacologically indistinguishable and have identical functional properties. Expression of AT1A and AT1B receptor genes are, however, differentially regulated. The AT1 receptor is ubiquitously expressed, including vascular smooth muscle, liver, kidney, heart, lung, adrenal cortex, pituitary and brain. Mutations disrupting AT1 receptor functions are not known, however genetic variants of the AGTR1 gene resulting from single nucleotide polymorphisms in the non-coding regions are documented. Studies aimed at finding significant association of AGTR1 gene variants with human pathological conditions in different populations are currently underway.
The molecular mass of human AT1 receptor is ≈41kDa and contains three N-glycosylation sites, eight phosphorylation sites, and six cysteine residues. The three-dimensional structure of the AT1 receptor is maintained by two disulphide bridges [24,28,55,58]. High-affinity binding of Ang II is determined by specific amino acid residues located on or near the extracellular regions of the receptor, as well as by residues in the transmembrane domain. These residues are probably in close proximity in the native receptor and held together by interactions between 7TM helices, the inter helical loops and the disulphide bridges [3,24,26,58]. Experimentally mapped site for binding of non-peptide antagonists such as losartan, EXP3174, candesartan and olmesartan overlaps with the membrane embedded portion of the Ang II binding pocket involving TMIII-VII [3,26,53,58,65,76]. Specific residues in TMIII, TMV, TMVI and TMVII of the AT1 receptor directly interact with both peptide and non-peptide ligands accounting for direct competitive binding which is experimentally observed. A class of AT1 receptor selective insurmountable non-peptide antagonists reversibly interact with the same membrane embedded residues [76]. Agonist binding to the AT1 receptor is reduced by GTP, suggesting the existence of heterogeneous states of receptor affinity due to association with G proteins. Selective signalling analogues of Ang II, which activate G protein independent AT1 receptor functions with low or zero efficacy for G protein mediated functions are described, but their mechanism of AT1 receptor activation is yet to be fully elucidated [38,78,85].
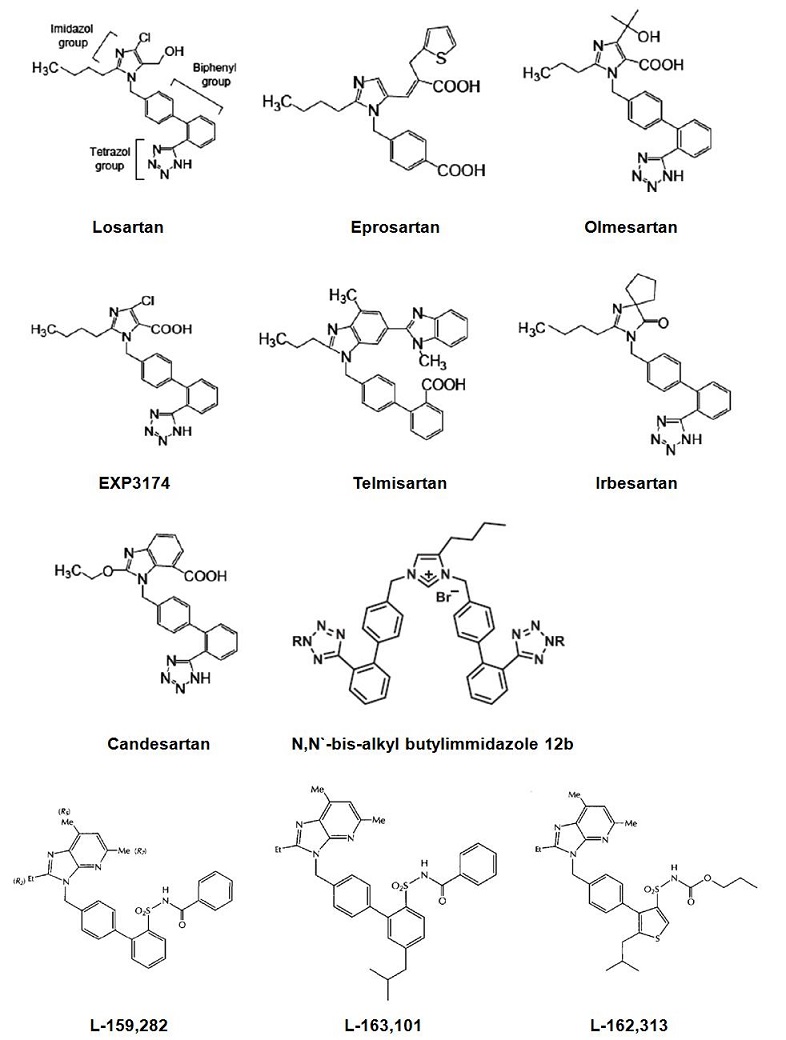
Figure 1. AT1 receptor agonists and antagonists
Signal transduction
Agonist binding to the AT1 receptor leads to the recruitment of heterotrimeric G proteins (Gq/G11 and/or Gi/Go) and/or adaptor proteins such as β-arrestin, GIT1, CARMA and/or kinases such as JAK2, pp60-src, GRK2 [9,16,33]. The stimulation of multiple intracellular signalling pathways within a cell or different signalling events induced by Ang II in different tissues occur in multiple phases, in seconds, minutes or hours, and involve the selective activation of multiple pathways over time. Classically, the signal transduction mechanisms of the AT1 receptor depend on different effectors: phospholipase (PL) C (formation of Inositol(1,4,5)P3 and DAG); voltage-dependent Ca2+ channels; PLD (cleavage of phosphatidylcholine); PLA2 (formation of prostaglandins and prostanoids); and adenylate cyclase (decrease in cAMP production). Cell specific effects have been described. For instance, in smooth muscle and some epithelial cells Ang II, like many growth factors, promotes activation of tyrosine-kinases such as pp60src and the JAK--STAT pathway [33,66]. The β-arrestin2 recruited to the AT1 receptor mediates activation of Ser/Thr kinases such as mitogen-activated protein (MAP) kinase spatially restricted to the cytoplasmic compartment [12,78]. Adaptor proteins such as GIT1 and CARMA1 recruited by the AT1 receptor activate, respectively, big MAPK (ERK5) and NFkB. Long-term activation of the NADH-NADPH oxidase pathway has also been implicated in gene induction and cell growth.
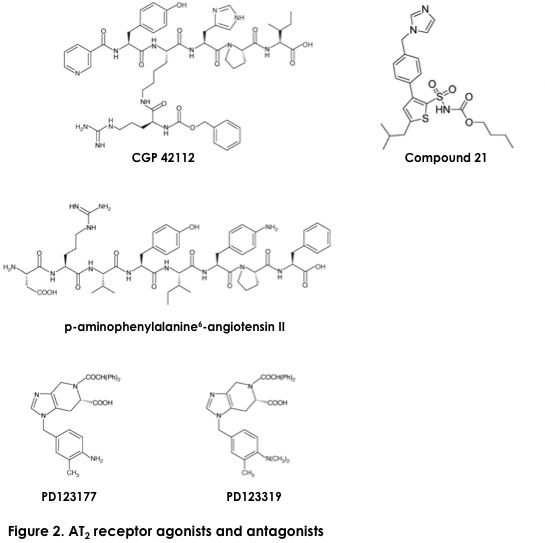
The AT2 Receptor
The gene coding for the AT2 receptor is located on chromosome X [46]. Unlike the AT1 receptor, no additional types or splice variants of the AT2 receptor have been reported in either man or rodents. The AT2 receptor has a molecular mass of ≈41kDa. The receptor contains five N-glycosylation sites, five phosphorylation sites, and 14 cysteine residues [43,54]. The cysteine residues in the extracellular region of AT2 receptor form two disulphide bonds. Both affinity and avidity of the AT2 receptor for ligands increases when reducing agents break one of the two disulphide bonds [24,58]. There is also a lack of effect of GTP analogues upon ligand-binding to AT2 sites. Expression of the AT2 receptor is developmentally regulated: it is highly expressed in various foetal tissues and at a lower density in adult adrenal medulla, brain, and reproductive tissues [16,32]. It appears to be re-expressed or up-regulated after vascular injury, myocardial infarction, cardiac failure or wound healing, possibly reflecting re-activation of a foetal genetic programme [21,50,52,57]. Preclinical in vitro and in vivo studies indicated that the AT2 receptor counterbalances the effect of the AT1 receptor [50,52,57,72,80]. Recent studies using the novel AT2 receptor non-peptide agonist for intervention in spinal cord injuries demonstrate a potential role beyond blood pressure regulation [56]. Genetic variants of the AGTR2 gene resulting from single nucleotide polymorphisms in the non-coding regions are also known. Mutations disrupting AT2 receptor functions are reported for X-linked mental retardation and related disorders [5,51,82]. In general, reports of mutations in the AGTR2 locus are more than mutations in the AGTR1 locus.
Signal transduction
The signal transduction mechanism of the AT2 receptor is still poorly understood. Although the sequence mofifs such as 'DRY' and NPxxY involved in G protein-activation of GPCRs are retained, agonist stimulation of the AT2 receptor does not incude an increase in cAMP, InsP3 and DAG formation [20,84]. The AT2 receptor does not undergo agonist-induced receptor phosphorylation [25]. A Giα 2-3 protein susceptible to pertussis toxin has been reported to participate in the signal transduction mechanism of the AT2 receptor [36,89]. Depending on the tissues, activation of the AT2 receptor appears to stimulate intracellular mechanisms involving Tyr and Ser/Thr phosphatases such as MKP-1, SHP-1 and PP2A, leading to the inactivation of the AT1 receptor- and growth factor- activated kinases [6,17,23,39,59,69,88]. As a consequence, there is an inactivation of MAP kinase, antiproliferation, promotion of apoptosis, repolarization trough opening of delayed-rectifier K+ channels and calcium and voltage activated potassium channel, closing of T -type Ca2+ channels and vasodilation [4,7,13,19,27,44,67,86-88,90]. The phosphatase activity is controlled by a cellular redox mechanism [62,71] involving bradykinin, nitric oxide and cGMP formation [29,47,68,79]. Through its phosphatase activity, the AT2 receptor regulates the NFκB pathway [61,63] and interferes with the inflammatory process [86-87]. Experimental studies suggest a protective action of AT2 receptor in tissue repair and regeneration. the c-kit(+)AT2(+) progenitor cell population has been identified in rat heart and bone marrow, which increases after induction of myocardial infarction. The AT2 receptor mediates cardiac homing of the c-kit(+) progenitor cells and promote repair of infarct tissue. Several modalities can result in AT2 receptor stimulation. For instance, AT1 receptor blockers can directly increase more angiotensin available to AT2 receptor. The AT2 receptor is constitutively active, hence inhibition of AT1 receptor can indirectly unmask hidden effects of AT2 receptor is constitutive activity. When inducing cell differentiation, the AT2 receptor can also stimulate MAP kinases Erk1/Erk2 [73].
The AT4 Receptor
The AT4 receptor has been identified by molecular cloning, pharmacological characterization of cloned receptor. The AT4 receptor is a class II transmembrane protein which is also known as insulin regulated aminopeptidase (IRAP), and oxytocinase (OTase) [1,81]. Ang 3-8 (Ang IV) binds selectively, reversibly, saturably and with high affinity (Kd 1 nM) to the IRAP/AT4 receptor [14], which has very low affinity for Ang II and for the AT1 and AT2 receptor antagonists. This "receptor" is widely distributed in brain and peripheral organs such as heart, vessels, adrenals, kidney, colon and prostate [14] Stable synthetic analogues of Ang IV such as Norleucine 1-Ang IV and divalinal-Ang IV act as AT4 receptor agonist and antagonist ligands, respectively [14]. LVV-haemorphin is an endogenous ligand for the AT4 receptor [1]. Ang IV and LVV-haemorphin compete for the binding of 125I-Nle1-Ang IV in IRAP-transfected HEK293 cells with IC50 values in the nanomolar range [1]. In membranes from tissue, covalent binding studies on AT4 binding sites have consistently revealed the presence of a 165 kDa peptide similar to that of IRAP [1,81].
OTase/IRAP is a type II integral membrane protein, homologous to aminopeptidases A, N, and other Zn2+-dependent aminopeptidases. It has a short intracellular domain, a single transmembrane-spanning domain and a large extracellular domain containing the catalytic site. It colocalized with Glut-4 vesicles. Ang IV inhibits the activity of OTase/IRAP and thereby reduces the processing of other bioactive peptides such as oxytocin, metenkephalin, dynorphin, neuromedin.
The Zn-binding state of IRAP might be a key factor in function of the protein as aminopeptidase and/or a signal transducing receptor, the AT4 receptor. The Zn-bound and apo-forms of IRAP have distinct pharmacological profiles [18]. Knowledge of the AT4 receptor-signalling pathway is incomplete. The AT4 receptor does not seem to be coupled to a G protein [55]. Whether the AT4 binding domain functions as a receptor as well as an enzyme regulatory site has yet to be determined.
It is likely that Ang IV has a physiological role in various functions such as cognition, cardiovascular and renal metabolism, and also in pathological conditions such as diabetes and hypertension. Ang IV binding to the AT4 receptor may be promoting the release of vasodilators such as nitric oxide, causing collagen accumulation in hypertrophied heart, controlling sodium transport in the kidney [28]. Pharmacological studies link the IRAP/AT4 receptor to enhanced learning and memory in normal rodents and reversal of the memory deficits seen in animal models of amnesia and possible involvement in cognition. Through binding Ang IV and hemorphin IRAP/AT4 receptor may enhance spatial working memory through enhanced hippocampal glucose uptake or blood flow. Ang IV may act through the inhibition of the activity of IRAP to reduce the degradation of oxytocin at the spinal cord, thereby leading to anti-hyperalgesia. The antidepressant-like effect of oxytocin is absent in IRAP/AT4 receptor-null mice [11,48]. Functional interaction between AT4 and the adenosine receptors may be involved in generalized seizure generation [49]. In conclusion, the IRAP/AT4 receptor has many receptor-like qualities in terms of ligand affinity but also has many enzymatic characteristics.
References
1. Albiston AL, McDowall SG, Matsacos D, Sim P, Clune E, Mustafa T, Lee J, Mendelsohn FA, Simpson RJ, Connolly LM et al.. (2001) Evidence that the angiotensin IV (AT(4)) receptor is the enzyme insulin-regulated aminopeptidase. J Biol Chem, 276 (52): 48623-6. [PMID:11707427]
2. Albiston AL, Mustafa T, McDowall SG, Mendelsohn FA, Lee J, Chai SY. (2003) AT4 receptor is insulin-regulated membrane aminopeptidase: potential mechanisms of memory enhancement. Trends Endocrinol Metab, 14 (2): 72-7. [PMID:12591177]
3. Baleanu-Gogonea C, Karnik S. (2006) Model of the whole rat AT1 receptor and the ligand-binding site. J Mol Model, 12 (3): 325-37. [PMID:16404618]
4. Barber MN, Sampey DB, Widdop RE. (1999) AT(2) receptor stimulation enhances antihypertensive effect of AT(1) receptor antagonist in hypertensive rats. Hypertension, 34 (5): 1112-6. [PMID:10567191]
5. Bienvenu T, Poirier K, Van Esch H, Hamel B, Moraine C, Fryns JP, Ropers HH, Beldjord C, Yntema HG, Chelly J. (2003) Rare polymorphic variants of the AGTR2 gene in boys with non-specific mental retardation. J Med Genet, 40 (5): 357-9. [PMID:12746399]
6. Brede M, Hadamek K, Meinel L, Wiesmann F, Peters J, Engelhardt S, Simm A, Haase A, Lohse MJ, Hein L. (2001) Vascular hypertrophy and increased P70S6 kinase in mice lacking the angiotensin II AT(2) receptor. Circulation, 104 (21): 2602-7. [PMID:11714657]
7. Buisson B, Laflamme L, Bottari SP, de Gasparo M, Gallo-Payet N, Payet MD. (1995) A G protein is involved in the angiotensin AT2 receptor inhibition of the T-type calcium current in non-differentiated NG108-15 cells. J Biol Chem, 270 (4): 1670-4. [PMID:7829501]
8. Bumpus FM, Catt KJ, Chiu AT, DeGasparo M, Goodfriend T, Husain A, Peach MJ, Taylor Jr DG, Timmermans PB. (1991) Nomenclature for angiotensin receptors. A report of the Nomenclature Committee of the Council for High Blood Pressure Research. Hypertension, 17 (5): 720-1. [PMID:2022414]
9. Catt KJ, Sandberg K, Balla T. (1993) Angiotensin II receptor and signal transduction mechanisms. In Cellular and molecular biology of the renin-angiotensin system Edited by Raizada MK, Phillips MI, Sumners C (CRC Press) 307-356. [ISBN:0849346223]
10. Chaki S, Inagami T. (1992) A newly found angiotensin II receptor subtype mediates cyclic GMP formation in differentiated Neuro-2A cells. Eur J Pharmacol, 225 (4): 355-6. [PMID:1323479]
11. Chow LH, Tao PL, Chen JC, Liao RM, Chang EP, Huang EY. (2013) A possible correlation between oxytocin-induced and angiotensin IV-induced anti-hyperalgesia at the spinal level in rats. Peptides, 39: 21-8. [PMID:23142109]
12. Christensen GL, Kelstrup CD, Lyngsø C, Sarwar U, Bøgebo R, Sheikh SP, Gammeltoft S, Olsen JV, Hansen JL. (2010) Quantitative phosphoproteomics dissection of seven-transmembrane receptor signaling using full and biased agonists. Mol Cell Proteomics, 9 (7): 1540-53. [PMID:20363803]
13. Cui T, Nakagami H, Iwai M, Takeda Y, Shiuchi T, Daviet L, Nahmias C, Horiuchi M. (2001) Pivotal role of tyrosine phosphatase SHP-1 in AT2 recepor-mediated apoptosis in rat foetal vascular smooth muscle cell. Cardiovasc Res, 49: 863-871. [PMID:11230986]
14. de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. (2000) International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev, 52 (3): 415-72. [PMID:10977869]
15. de Gasparo M, Husain A, Alexander W, Catt KJ, Chiu AT, Drew M, Goodfriend T, Harding JW, Inagami T, Timmermans PB. (1995) Proposed update of angiotensin receptor nomenclature. Hypertension, 25 (5): 924-7. [PMID:7737728]
16. de Gasparo MBottari. (1995) Characteristics of angiotensin II receptors and their role in cell and organ physiology. In Hypertension: Pathophysiology, Diagnosis, and Management Edited by Laragh JH, Brenner BM (Raven Press Ltd.) 1695-1720. [ISBN:0781701570]
17. De Paolis P, Porcellini A, Savoia C, Lombardi A, Gigante B, Frati G, Rubattu S, Musumeci B, Volpe M. (2002) Functional cross-talk between angiotensin II and epidermal growth factor receptors in NIH3T3 fibroblasts. J Hypertens, 20 (4): 693-9. [PMID:11910305]
18. Demaegdt H, Lukaszuk A, De Buyser E, De Backer JP, Szemenyei E, Tóth G, Chakravarthy S, Panicker M, Michotte Y, Tourwé D et al.. (2009) Selective labeling of IRAP by the tritiated AT(4) receptor ligand [3H]Angiotensin IV and its stable analog [3H]AL-11. Mol Cell Endocrinol, 311 (1-2): 77-86. [PMID:19643163]
19. Dimitropoulou C, White RE, Fuchs L, Zhang H, Catravas JD, Carrier GO. (2001) Angiotensin II relaxes microvessels via the AT(2) receptor and Ca(2+)-activated K(+) (BK(Ca)) channels. Hypertension, 37 (2): 301-7. [PMID:11230289]
20. Dudley DT, Hubbell SE, Summerfelt RM. (1991) Characterization of angiotensin II (AT2) binding sites in R3T3 cells. Mol Pharmacol, 40 (3): 360-7. [PMID:1896025]
21. Dzau VJ, Horiuchi M. (1996) Differential expression of angiotensin receptor subtypes in the myocardium: a hypothesis. Eur Heart J, 17 (7): 978-80. [PMID:8809509]
22. Dzau VJ, Mukoyama M, Pratt RE. (1994) Molecular biology of angiotensin receptors: target for drug research?. J Hypertens Suppl, 12 (2): S1-5. [PMID:7965260]
23. Eguchi S, Dempsey PJ, Frank GD, Motley ED, Inagami T. (2001) Activation of MAPKs by angiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p38 MAPK but not for JNK. J Biol Chem, 276 (11): 7957-62. [PMID:11116149]
24. Feng YH, Saad Y, Karnik SS. (2000) Reversible inactivation of AT(2) angiotensin II receptor from cysteine-disulfide bond exchange. FEBS Lett, 484 (2): 133-8. [PMID:11068047]
25. Feng YH, Sun Y, Douglas JG. (2002) Gbeta gamma -independent constitutive association of Galpha s with SHP-1 and angiotensin II receptor AT2 is essential in AT2-mediated ITIM-independent activation of SHP-1. Proc Natl Acad Sci USA, 99 (19): 12049-54. [PMID:12221292]
26. Fillion D, Cabana J, Guillemette G, Leduc R, Lavigne P, Escher E. (2013) Structure of the human angiotensin II type 1 (AT1) receptor bound to angiotensin II from multiple chemoselective photoprobe contacts reveals a unique peptide binding mode. J Biol Chem, 288 (12): 8187-97. [PMID:23386604]
27. Fischer TA, Singh K, O'Hara DS, Kaye DM, Kelly RA. (1998) Role of AT1 and AT2 receptors in regulation of MAPKs and MKP-1 by Ang II in adult cardiac myocytes. Am J Physiol, 275: H906-H916. [PMID:9724295]
28. Geerts H, de Brabander M, Nuydens R. (1991) Nanovid microscopy. Nature, 351 (6329): 765-6. [PMID:1712078]
29. Gohlke P, Pees C, Unger T. (1998) AT2 receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin-dependent mechanism. Hypertension, 31 (1 Pt 2): 349-55. [PMID:9453327]
30. Gold S, Haran I, Attias J, Shapira I, Shahar A. (1989) Biochemical and cardiovascular measures in subjects with noise-induced hearing loss. J Occup Med, 31 (11): 933-7. [PMID:2809800]
31. Goodfriend TL, Elliott ME, Catt KJ. (1996) Angiotensin receptors and their antagonists. N Engl J Med, 334 (25): 1649-54. [PMID:8628362]
32. Griendling KK, Lassègue B, Alexander RW. (1996) Angiotensin receptors and their therapeutic implications. Annu Rev Pharmacol Toxicol, 36: 281-306. [PMID:8725391]
33. Griendling KK, Ushiofukai M, Lassegue B, Alexander RW. (1997) Angiotensin II signaling in vascular smooth muscle - new concepts. Hypertension, 29: 366-373. [PMID:9039129]
34. Hallberg M. (2009) Targeting the insulin-regulated aminopeptidase/AT4 receptor for cognitive disorders. Drug News Perspect, 22 (3): 133-9. [PMID:19440555]
35. Hanley MR, Cheung WT, Hawkins P, Poyner D, Benton HP, Blair L, Jackson TR, Goedert M. (1990) The mas oncogene as a neural peptide receptor: expression, regulation and mechanism of action. Ciba Found Symp, 150: 23-38; discussion 38-46. [PMID:2197067]
36. Hansen JL, Servant G, Baranski TJ, Fujita T, Iiri T, Sheikh SP. (2000) Functional reconstitution of the angiotensin II type 2 receptor and G(i) activation. Circ Res, 87 (9): 753-9. [PMID:11055978]
37. Harding JW, Cook VI, Miller-Wing AV, Hanesworth JM, Sardinia MF, Hall KL, Stobb JW, Swanson GN, Coleman JK, Wright JW et al.. (1992) Identification of an AII(3-8) [AIV] binding site in guinea pig hippocampus. Brain Res, 583 (1-2): 340-3. [PMID:1504842]
38. Holloway AC, Qian H, Pipolo L, Ziogas J, Miura S, Karnik S, Southwell BR, Lew MJ, Thomas WG. (2002) Side-chain substitutions within angiotensin II reveal different requirements for signaling, internalization, and phosphorylation of type 1A angiotensin receptors. Mol Pharmacol, 61 (4): 768-77. [PMID:11901215]
39. Horiuchi M, Hayashida W, Kambe T, Yamada T, Dzau VJ. (1997) Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J Biol Chem, 272 (30): 19022-6. [PMID:9228085]
40. Inagami T, Iwai N, Sasaki K, Guo DF, Furuta H, Yamano Y, Bardhan S, Chaki S, Makito N, Badr K. (1993) Angiotensin II receptors: cloning and regulation. Arzneimittelforschung, 43 (2A): 226-8. [PMID:8498969]
41. Inagami T, Iwai N, Sasaki K, Yamano Y, Bardhan S, Chaki S, Guo DF, Furuta H, Ohyama K, Kambayashi Y et al.. (1994) Cloning, expression and regulation of angiotensin II receptors. Eur Heart J, 15 Suppl D: 104-7. [PMID:7713098]
42. Jaiswal N, Tallant EA, Jaiswal RK, Diz DI, Ferrario CM. (1993) Differential regulation of prostaglandin synthesis by angiotensin peptides in porcine aortic smooth muscle cells: subtypes of angiotensin receptors involved. J Pharmacol Exp Ther, 265 (2): 664-73. [PMID:8496814]
43. Kambayashi Y, Bardhan S, Takahashi K, Tsuzuki S, Inui H, Hamakubo T, Inagami T. (1993) Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J Biol Chem, 268 (33): 24543-6. [PMID:8227011]
44. Kang J, Richards EM, Posner P, Sumners C. (1995) Modulation of the delayed rectifier K+ current in neurons by an angiotensin II type 2 receptor fragment. Am J Physiol, 268 (1 Pt 1): C278-82. [PMID:7840157]
45. Kemp BA, Bell JF, Rottkamp DM, Howell NL, Shao W, Navar LG, Padia SH, Carey RM. (2012) Intrarenal angiotensin III is the predominant agonist for proximal tubule angiotensin type 2 receptors. Hypertension, 60 (2): 387-95. [PMID:22689743]
46. Koike G, Horiuchi M, Yamada T, Szpirer C, Jacob HJ, Dzau VJ. (1994) Human type 2 angiotensin II receptor gene
47. Kurisu S, Ozono R, Oshima T, Kambe M, Ishida T, Sugino H, Matsuura H, Chayama K, Teranishi Y, Iba O et al.. (2003) Cardiac angiotensin II type 2 receptor activates the kinin/NO system and inhibits fibrosis. Hypertension, 41 (1): 99-107. [PMID:12511537]
48. Loyens E, De Bundel D, Demaegdt H, Chai SY, Vanderheyden P, Michotte Y, Gard P, Smolders I. (2013) Antidepressant-like effects of oxytocin in mice are dependent on the presence of insulin-regulated aminopeptidase. Int J Neuropsychopharmacol, 16 (5): 1153-63. [PMID:23177092]
49. Loyens E, Schallier A, Chai SY, De Bundel D, Vanderheyden P, Michotte Y, Smolders I. (2011) Deletion of insulin-regulated aminopeptidase in mice decreases susceptibility to pentylenetetrazol-induced generalized seizures. Seizure, 20 (8): 602-5. [PMID:21612947]
50. Matsubara H. (1998) Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ Res, 83 (12): 1182-91. [PMID:9851935]
51. Maul B, von Bohlen und Halbach O, Becker A, Sterner-Kock A, Voigt JP, Siems WE, Grecksch G, Walther T. (2008) Impaired spatial memory and altered dendritic spine morphology in angiotensin II type 2 receptor-deficient mice. J Mol Med, 86 (5): 563-71. [PMID:18335189]
52. Millatt LJ, Abdel-Rahman EM, Siragy HM. (1999) Angiotensin II and nitric oxide: a question of balance. Regul Pept, 81 (1-3): 1-10. [PMID:10395403]
53. Miura S, Karnik SS, Saku K. (2011) Review: angiotensin II type 1 receptor blockers: class effects versus molecular effects. J Renin Angiotensin Aldosterone Syst, 12 (1): 1-7. [PMID:20603272]
54. Mukoyama M, Nakajima M, Horiuchi M, Sasamura H, Pratt RE, Dzau VJ. (1993) Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J Biol Chem, 268 (33): 24539-42. [PMID:8227010]
55. Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE. (1991) Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature, 351 (6323): 233-6. [PMID:2041570]
56. Namsolleck P, Boato F, Schwengel K, Paulis L, Matho KS, Geurts N, Thöne-Reineke C, Lucht K, Seidel K, Hallberg A et al.. (2013) AT2-receptor stimulation enhances axonal plasticity after spinal cord injury by upregulating BDNF expression. Neurobiol Dis, 51: 177-91. [PMID:23174180]
57. Nazzaro P, Manzari M, Merlo M, Triggiani R, Scarano A, Ciancio L, Pirrelli A. (1999) Distinct and combined vascular effects of ACE blockade and HMG-CoA reductase inhibition in hypertensive subjects. Hypertension, 33 (2): 719-25. [PMID:10024335]
58. Noda K, Saad Y, Kinoshita A, Boyle TP, Graham RM, Husain A, Karnik SS. (1995) Tetrazole and carboxylate groups of angiotensin receptor antagonists bind to the same subsite by different mechanisms. J Biol Chem, 270 (5): 2284-9. [PMID:7530721]
59. Nouet S, Nahmias C. (2000) Signal transduction from the angiotensin II AT2 receptor. Trends Endocrinol Metab, 11 (1): 1-6. [PMID:10652498]
60. Padia SH, Kemp BA, Howell NL, Fournie-Zaluski MC, Roques BP, Carey RM. (2008) Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension, 51 (2): 460-5. [PMID:18158338]
61. Reinecke K, Lucius R, Reinecke A, Rickert U, Herdegen T, Unger T. (2003) Angiotensin II accelerates functional recovery in the rat sciatic nerve in vivo: role of the AT2 receptor and the transcription factor NF-kappaB. FASEB J, 17 (14): 2094-6. [PMID:14500552]
62. Rueckschloss U, Quinn MT, Holtz J, Morawietz H. (2002) Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler Thromb Vasc Biol, 22 (11): 1845-51. [PMID:12426214]
63. Sadoshima J. (2000) Cytokine actions of angiotensin II. Circ Res, 86 (12): 1187-9. [PMID:10864905]
64. Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M et al.. (2003) Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA, 100 (14): 8258-63. [PMID:12829792]
65. Saulière A, Bellot M, Paris H, Denis C, Finana F, Hansen JT, Altié MF, Seguelas MH, Pathak A, Hansen JL et al.. (2012) Deciphering biased-agonism complexity reveals a new active AT1 receptor entity. Nat Chem Biol, 8 (7): 622-30. [PMID:22634635]
66. Sayeski PP, Ali MS, Semeniuk DJ, Doan TN, Bernstein KE. (1998) Angiotensin II signal transduction pathways. Regul Pept, 78 (1-3): 19-29. [PMID:9879743]
67. Silvestre JS, Tamarat R, Senbonmatsu T, Icchiki T, Ebrahimian T, Iglarz M, Besnard S, Duriez M, Inagami T, Lévy BI. (2002) Antiangiogenic effect of angiotensin II type 2 receptor in ischemia-induced angiogenesis in mice hindlimb. Circ Res, 90 (10): 1072-9. [PMID:12039796]
68. Siragy HM, El-Kersh MA, De Gasparo M, Webb RL, Carey RM. (2002) Differences in AT2 -receptor stimulation between AT1 -receptor blockers valsartan and losartan quantified by renal interstitial fluid cGMP. J Hypertens, 20 (6): 1157-63. [PMID:12023686]
69. Siragy HM, Inagami T, Ichiki T, Carey RM. (1999) Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Natl Acad Sci USA, 96 (11): 6506-10. [PMID:10339618]
70. Smith RD, Timmermans PB. (1994) Human angiotensin receptor subtypes. Curr Opin Nephrol Hypertens, 3 (1): 112-22. [PMID:7850406]
71. Sohn HY, Raff U, Hoffmann A, Gloe T, Heermeier K, Galle J, Pohl U. (2000) Differential role of angiotensin II receptor subtypes on endothelial superoxide formation. Br J Pharmacol, 131 (4): 667-72. [PMID:11030714]
72. Stoll M, Steckelings UM, Bottari SP, Paul M, Metzger R, Unger T. (1995) The Angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest, 95: 651-657. [PMID:7860748]
73. Stroth U, Blume A, Mielke K, Unger T. (2000) Angiotensin AT(2) receptor stimulates ERK1 and ERK2 in quiescent but inhibits ERK in NGF-stimulated PC12W cells. Brain Res Mol Brain Res, 78 (1-2): 175-80. [PMID:10891597]
74. Sugiura N, Hagiwara H, Hirose S. (1992) Molecular cloning of porcine soluble angiotensin-binding protein. J Biol Chem, 267 (25): 18067-72. [PMID:1517239]
75. Swindle JD, Santos KL, Speth RC. (2013) Pharmacological characterization of a novel non-AT1, non-AT2 angiotensin binding site identified as neurolysin. Endocrine, 44 (2): 525-31. [PMID:23412923]
76. Takezako T, Gogonea C, Saad Y, Noda K, Karnik SS. (2004) "Network leaning" as a mechanism of insurmountable antagonism of the angiotensin II type 1 receptor by non-peptide antagonists. J Biol Chem, 279 (15): 15248-57. [PMID:14754891]
77. Tang SS, Rogg H, Schumacher R, Dzau VJ. (1992) Characterization of nuclear angiotensin-II-binding sites in rat liver and comparison with plasma membrane receptors. Endocrinology, 131 (1): 374-80. [PMID:1612017]
78. Thomas WG, Qian H, Chang CS, Karnik S. (2000) Agonist-induced phosphorylation of the angiotensin II (AT(1A)) receptor requires generation of a conformation that is distinct from the inositol phosphate-signaling state. J Biol Chem, 275 (4): 2893-900. [PMID:10644757]
79. Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasawa S, Takai S, Miyazaki M, Nozawa Y, Ozono R, Nakagawa K et al.. (1999) Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest, 104 (7): 925-35. [PMID:10510333]
80. Unger T. (1999) The angiotensin type 2 receptor: variations on an enigmatic theme. J Hypertens, 17 (12 Pt 2): 1775-86. [PMID:10703869]
81. Vauquelin G, Michotte Y, Smolders I, Sarre S, Ebinger G, Dupont A, Vanderheyden P. (2002) Cellular targets for angiotensin II fragments: pharmacological and molecular evidence. J Renin Angiotensin Aldosterone Syst, 3 (4): 195-204. [PMID:12584663]
82. Vervoort VS, Beachem MA, Edwards PS, Ladd S, Miller KE, de Mollerat X, Clarkson K, DuPont B, Schwartz CE, Stevenson RE et al.. (2002) AGTR2 mutations in X-linked mental retardation. Science, 296 (5577): 2401-3. [PMID:12089445]
83. Wangler NJ, Santos KL, Schadock I, Hagen FK, Escher E, Bader M, Speth RC, Karamyan VT. (2012) Identification of membrane-bound variant of metalloendopeptidase neurolysin (EC 3.4.24.16) as the non-angiotensin type 1 (non-AT1), non-AT2 angiotensin binding site. J Biol Chem, 287 (1): 114-22. [PMID:22039052]
84. Webb ML, Liu EC, Cohen RB, Hedberg A, Bogosian EA, Monshizadegan H, Molloy C, Serafino R, Moreland S, Murphy TJ et al.. (1992) Molecular characterization of angiotensin II type II receptors in rat pheochromocytoma cells. Peptides, 13 (3): 499-508. [PMID:1326103]
85. Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. (2003) Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA, 100 (19): 10782-7. [PMID:12949261]
86. Wu L, Iwai M, Nakagami H, Chen R, Suzuki J, Akishita M, de Gasparo M, Horiuchi M. (2002) Effect of angiotensin II type 1 receptor blockade on cardiac remodeling in angiotensin II type 2 receptor null mice. Arterioscler Thromb Vasc Biol, 22 (1): 49-54. [PMID:11788460]
87. Wu L, Iwai M, Nakagami H, Li Z, Chen R, Suzuki J, Akishita M, de Gasparo M, Horiuchi M. (2001) Roles of angiotensin II type 2 receptor stimulation associated with selective angiotensin II type 1 receptor blockade with valsartan in the improvement of inflammation-induced vascular injury. Circulation, 104 (22): 2716-21. [PMID:11723025]
88. Yamada T, Horiuchi M, Dzau VJ. (1996) Angiotensin II type 2 receptor mediates programmed cell death. Proc Natl Acad Sci USA, 93 (1): 156-60. [PMID:8552595]
89. Zhang J, Pratt RE. (1996) The AT2 receptor selectively associates with Gialpha2 and Gialpha3 in the rat fetus. J Biol Chem, 271 (25): 15026-33. [PMID:8663053]
90. Zimpelmann J, Burns KD. (2001) Angiotensin II AT(2) receptors inhibit growth responses in proximal tubule cells. Am J Physiol Renal Physiol, 281 (2): F300-8. [PMID:11457722]







