
GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
QRFP receptor: Introduction
Basic Characteristics
The human gene encoding the QRFPR, previously designated as an orphan GPCR receptor was identified in 2001 by Lee et al from a hypothalamus cDNA library [9]. However, the reported cDNA (AF411117) is a chimera with bases 1-127 derived from chromosome 1 and bases 155-1368 derived from chromosome 4. When corrected, QRFPR (also referred to as SP9155 or AQ27) encodes a 431 amino acid protein that shares sequence similarities in the transmembrane spanning regions with other peptide receptors. These include neuropeptide FF2 (38%), neuropeptide Y2 (37%) and galanin GalR1 (35%) receptors.
QRFPR exists as a single gene in the genome sequences of humans, cow, dog, and chicken. In both mouse and rat Qrfpr exists as two genes which have been referred to as A and B [11] although these are not the official gene names. The A genes encode proteins of 433 amino acids while the B genes encode proteins of 415 or 416 amino acids. Human QRFPR shares 85% amino acid identity with mouse QrfprA and 81% identity to mouse QrfprB while the two mouse genes have 76% identity. Whereas the human, dog, cow and chicken genes are syntenic, meaning that the flanking genes are the same and in the same order, the mouse and rat genes are only partially syntenic. The genes immediately centromeric of the human QRFPR gene (MAD2L1, PRDM5, c4orf31, TNIP3) flank the mouse QrfprB gene at 6qC1 whereas the genes immediately teleomeric of human QRFPR (ANXA5, EXOSC9, TRPC3) flank the mouse QrfprA at 3qB. Thus it appears that there was an evolutionary chromosomal translocation in the immediate vicinity of the QRFPR gene that gave rise to two genes in the rodents and a single gene in the other species. Since the chicken lineage diverged first, the simplest explanation is that a rearrangement occurred in the rodent lineage resulting in a duplication of QRFPR. The official names of the mouse and rat A genes are Qrfpr while the B genes are C130060K24Rik for mouse and RGD1560028 for rat. In 2003, the QRFPR was linked with its cognate peptide, a 26-amino-acid RF-amide peptide, QRFP26 (also called P518 and 26Rfamide) by Jiang et al (2006, [7]). The DNA sequence encoding QRFP26 has been found to also encode an N-terminally elongated form of the peptide, with 43 amino acids, QRFP43. CHO cells transfected with human QRFP peptide cDNA are found to produce both QRFP43 and QRFP26 [6-7] and CHO cells transfected with human QRFPR respond to QRFP43 and QRFP26, with QRFP43 being the more potent of the two forms [6]. Additionally, results from such experiments have suggested that QRFPR couples to G proteins, Gi/o and Gq [6].
Receptor Distribution
mRNA expression
Northern blot analysis of human tissue has detected QRFPR mRNA transcripts predominantly in the brain, particularly in the thalamus, hypothalamus and pituitary. In addition, QRFPR transcripts were also detected in the frontal and occipital cortices, basal forebrain, midbrain and pons [9]. This was confirmed using quantitative PCR, where both human QRFPR and the QFRP peptide precursor mRNAs exhibited highest expression in brain. Coordinate expression of receptor and ligand mRNAs occurred in most brain regions, however, in peripheral tissues receptor was nonexistent or expressed at low levels. In brain, receptor is most abundant in retina, trigeminal ganglion, hypothalamus, and vestibular nucleus, whereas peptide mRNA is most abundant in cerebellum, medulla, retina, and vestibular nucleus. In peripheral tissues, significant expression of human QRFPR receptor was found only in heart, kidney, and testes. The peptide precursor mRNA however was found in prostate, testes, colon, thyroid, parathyroid, coronary artery, and bladder [7]
In rat, quantitative RT-PCR revealed highest level of Qrfpra mRNA in the adrenal gland, high levels in the hypothalamus and moderate levels in the thalamus, midbrain, medulla oblongata, testis, and eye [6]. In situ hybridisation revealed strong signals in neurons within the piriform cortex, cortex-amygdala transition zone, ventral pallidum, lateral preoptic area, ventromedial hypothalamic nucleus, zona incerta, posterior hypothalamic area, marginal zone median geniculate, dorsal raphe nucleus, nucleus of the brachium inferior colliculus, intergeniculate leaf, locus caeruleus, and central gray part. In addition to high levels of mRNA expression in the adrenal gland [6].
In mouse, quantitative PCR revealed high QrfprA receptor expression in the brain, in particular the hypothalamus, cortex, and spinal cord [7]. Quantitative PCR has also revealed mRNA distributions of two forms of the Qrfpr, QrfprA and QrfprB, both of which are found in the CNS, eye, testis and adrenal gland while in situ hybridization shows little, if any, overlap in the distributions of QrfprA and QrfprB in brain [11].
Radioligand binding
In humans, quantitative autoradiography has been used to measure binding of [125I]-QRFP43 in human tissues from a range of organs including heart, lungs, kidney and adrenal. Tissue profiling reveals a remarkably discrete distribution amongst the tissues tested with highest densities of specific [125I]-QRFP43 binding to adrenals, predominately to the zona reticularis and glomerulosa [8]. In rat radioligand binding experiments have revealed that Qrfprs are particularly abundant in nuclei associated with feeding and arousal behavior, reproductive function, control of arterial blood pressure, and transmission and/or integration of olfactory, visual, and nociceptive stimuli.
QRFP family of peptides
QRFP26 is a novel 26-residue RFamide peptide, initially isolated from a frog brain extract by HPLC purification and characterized by mass spectrometry, that exhibits no meaningful sequence similarity with the other vertebrate RFamide peptides [3]. The only consistent characteristic in QRFP26 with other RFRPs is the Arg-Phe-NH2 motif. Additionally, QRFP26 is the only representative of the RFRP family with the Phe-Arg-Phe-NH2 sequence, in comparison to other vertebrate RFRPs which possess a Gln-Arg-Phe-NH2 (e.g. RFRP-3, NPFF, and neuropeptide AF), a Gly-Arg-Phe-NH2 (e.g. PrRP), or a Leu-Arg-Phe-NH2 (e.g. RFRP-1, GnIH and R-RFa) motif. QRFP26 is therefore indicated as a member of a previously uncharacterized subfamily of RFRPs identified in vertebrates [7]. The primary structures of human, rat, and frog QRFP26 was shown to exhibit approximately 80% identity, with full conservation of the C-terminal octapeptide from amphibians to mammals [3]. To date, the cDNA encoding the QRFP26 precursor has been identified in rat [3,6], mouse [6-7], ox [6] and human [3,6-7].
The peptide sequence of the QRFP26 and its N-terminally elongated form QRFP43 are shown below and in Figure 1. Amino acid sequences from human, mouse and rat have been aligned with differences from human highlighted in colour.

Figure 1 shows the human encoded preprotein of QRFP26 and QRFP43, 136 amino acids long encoding a signal peptide (SP) of 17 aa, a fully conserved downstream peptide of six residues followed by a dibasic processing site (Arg-Arg), and the QRFP26 sequence is located in the C-terminal region of the precursor. Human QRFP26 is seen to be composed of 26 aa, followed by a Gly residue. The peptide is also flanked, at its N terminus, by a monobasic residue (Lys) and followed by a dibasic cleavage site (Arg-Arg). A dibasic site within the QRFP26 sequence suggests that the peptide could be further processed to generate the highly conserved C-terminal peptide (Lys)-Gly-Gly-Phe-Ser-Phe-Arg-Phe-NH2, although isolation of QRFP26 from frog brain indicates inefficient cleavage. An additional RFRP of 9 aa, flanked by two Arg residues, is found upstream of the QRFP26. This nonapeptide has identical C terminal tripeptide sequence to QRFP26 indicating that it is likely to have come about from exon duplication of an ancestral DNA sequence. It has been suggested that the nonapeptide is generated in addition to QRFP26 by prohormone-convertase processing of the QRFP26 precursor as seen in other RFRPs, also flanked by individual Arg residues [7]. QRFP43 may be generated by cleavage by prohormone-convertases at Arg90. QRFP43 has also been shown to be produced efficiently in CHO cells transfected with pro-QRFP26 [6].
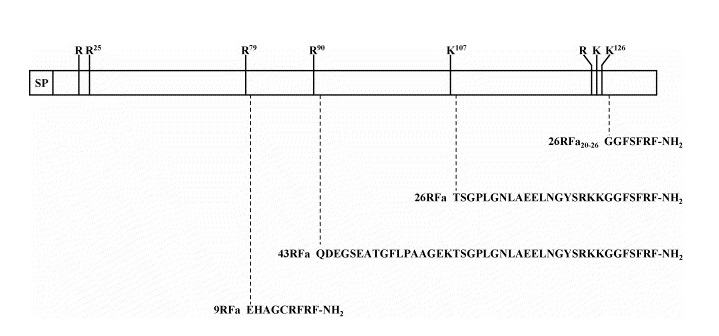
Figure 2: Schematic representation of human QRFP preprotein.
Potential mono/polybasic cleavage sites are indicated by vertical bars. The sequences of the four RFamide peptides that may be generated by processing at these cleavage sites are indicated. SP, signal peptide [4].
The two distinct molecular forms, QRFP26 and an N-terminally elongated form QRFP43, have been identified in human brain and spinal cord [3,11] and both peptide forms have been identified as the cognate ligands of the human former orphan GPCR, GPR103, now officially known as QRFPR [6-7,11].
Peptide Expression Pattern
mRNA expression analysis has revealed that the peptide QRFP26 precursor gene is predominantly expressed in various brain regions, coronary arteries, thyroid and parathyroid glands, large intestine, colon, bladder, testes, and prostate [3]. Through quantitative RT-PCR analysis it has been revealed that, in the rat brain, the gene encoding the QRFP26 precursor is strongly expressed in the hypothalamus compared to other regions of the brain [5,7]. In situ hybridization histochemistry has determined that QRFP26 mRNA is expressed primarily in the ventromedial hypothalamic nucleus, the arcuate nucleus, the lateral hypothalamic area, and the retrochiasmatic area [3,5,11].
Tissue Function
Using such observations, potential biological activities of QRFP26 have been explored. Studies show that i.c.v. administration of QRFP26 and QRFP43 stimulates food intake and causes obesity in mice [3-4,10-11]. Central injection of QRFP43 has been shown to stimulate locomotor activity [4,11] and elevate arterial blood pressure and heart rate [11]. QRFP26 has also been proposed to play a role peripherally, in bone formation, and Qrfpr-knockout mice have been seen to develop osteopenia [1].
Experiments on the rat however suggest that QRFP26 may activate receptors other than Qrfpr. Competition experiments have confirmed that QRFP26 interacts with an RFamide peptide receptor distinct from Qrfpr, thought to be NPFF2 and wide distribution of QRFP26 binding sites suggests that QRFP26 has multiple functions in the CNS that are mediated by at least two distinct receptors [2]. Note, however, that this analysis does not take into account the predicted QrfprB gene.
References
1. Baribault H, Danao J, Gupte J, Yang L, Sun B, Richards W, Tian H. (2006) The G-protein-coupled receptor GPR103 regulates bone formation. Mol Cell Biol, 26 (2): 709-17. [PMID:16382160]
2. Bruzzone F, Lectez B, Alexandre D, Jégou S, Mounien L, Tollemer H, Chatenet D, Leprince J, Vallarino M, Vaudry H et al.. (2007) Distribution of 26RFa binding sites and GPR103 mRNA in the central nervous system of the rat. J Comp Neurol, 503 (4): 573-91. [PMID:17534937]
3. Chartrel N, Bruzzone F, Dujardin C, Leprince J, Tollemer H, Anouar Y, Vallarino M, Costentin J, Vaudry H. (2005) Identification of 26RFa from frog brain: a novel hypothalamic neuropeptide with orexigenic activity in mammals. Ann N Y Acad Sci, 1040: 80-3. [PMID:15891009]
4. do Rego JC, Leprince J, Chartrel N, Vaudry H, Costentin J. (2006) Behavioral effects of 26RFamide and related peptides. Peptides, 27 (11): 2715-21. [PMID:16730856]
5. Fukusumi S, Fujii R, Hinuma S. (2006) Recent advances in mammalian RFamide peptides: the discovery and functional analyses of PrRP, RFRPs and QRFP. Peptides, 27 (5): 1073-86. [PMID:16500002]
6. Fukusumi S, Yoshida H, Fujii R, Maruyama M, Komatsu H, Habata Y, Shintani Y, Hinuma S, Fujino M. (2003) A new peptidic ligand and its receptor regulating adrenal function in rats. J Biol Chem, 278 (47): 46387-95. [PMID:12960173]
7. Jiang Y, Luo L, Gustafson EL, Yadav D, Laverty M, Murgolo N, Vassileva G, Zeng M, Laz TM, Behan J et al.. (2003) Identification and characterization of a novel RF-amide peptide ligand for orphan G-protein-coupled receptor SP9155. J Biol Chem, 278 (30): 27652-7. [PMID:12714592]
8. Kuc RE, Mitchell JD, Davenport AD. (2006) The novel ligand [125I]-QRFP43 reveals a remarkably discrete distribution of the orphan receptor GPR103 in human adrenal. Proceedings of the British Pharmacological Society, 4 (2): abst186.
9. Lee DK, Nguyen T, Lynch KR, Cheng R, Vanti WB, Arkhitko O, Lewis T, Evans JF, George SR, O'Dowd BF. (2001) Discovery and mapping of ten novel G protein-coupled receptor genes. Gene, 275 (1): 83-91. [PMID:11574155]
10. Moriya R, Sano H, Umeda T, Ito M, Takahashi Y, Matsuda M, Ishihara A, Kanatani A, Iwaasa H. (2006) RFamide peptide QRFP43 causes obesity with hyperphagia and reduced thermogenesis in mice. Endocrinology, 147 (6): 2916-22. [PMID:16543370]
11. Takayasu S, Sakurai T, Iwasaki S, Teranishi H, Yamanaka A, Williams SC, Iguchi H, Kawasawa YI, Ikeda Y, Sakakibara I et al.. (2006) A neuropeptide ligand of the G protein-coupled receptor GPR103 regulates feeding, behavioral arousal, and blood pressure in mice. Proc Natl Acad Sci USA, 103 (19): 7438-43. [PMID:16648250]







