
GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Metabotropic glutamate receptors: Introduction
General
Glutamate is considered the major excitatory neurotransmitter in the mammalian central nervous system (CNS), contributing excitatory input to as many as half of all central synapses. Glutamate transmission is primarily mediated by ion channel receptors, or 'ionotropic' glutamate receptors, which include NMDA, AMPA and kainate receptors. More recently, a novel family of structurally related G protein-coupled, or so called 'metabotropic' glutamate, receptors has been elucidated by pharmacological and molecular techniques. In general, metabotropic glutamate receptors bind glutamate and function to modulate or 'fine-tune' excitatory and inhibitory transmission by presynaptic, postsynaptic, and glial mechanisms (for reviews see refs. [2,6,8,12-15]).
Structural features
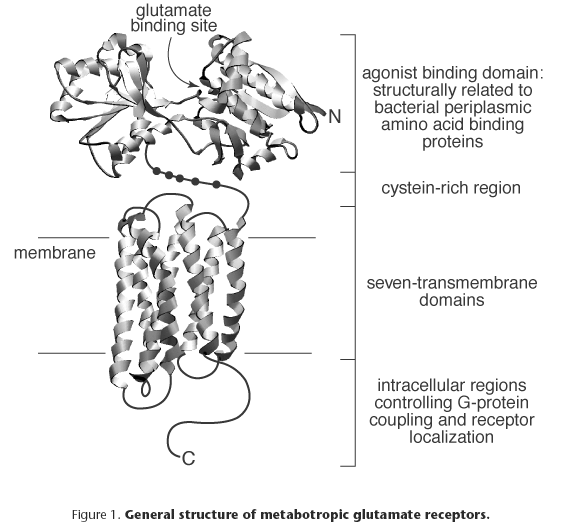
Metabotropic glutamate receptors (Figure 1) are characterized by a large N-terminal extracellular domain of ~560 amino acids (aa) which possesses the glutamate binding domain and confers selectivity for agonists. The ligand-binding domain shares structural similarity to bacterial periplasmic binding proteins[9]. However, certain amino acids downstream from the first transmembrane domain (TM) (e.g. in the C-terminal domain) might influence agonist affinity and potency. In contrast to other G protein-coupled receptors (e.g. those for catecholamines), where the third intracellular loop appears critical for G protein-coupling, in metabotropic glutamate receptors the second intracellular loop is important for this function and for determining the transduction mechanism[2].
Metabotropic glutamate receptor 'groups'
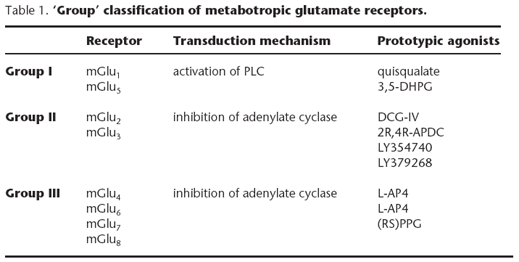
Based on the proposal of Nakanishi[8], metabotropic glutamate receptors have been categorised into three groups based on the degree of sequence homology (~70% homology within groups and ~40% homology between groups), signal transduction mechanisms when expressed in recombinant systems, and pharmacological properties (activation by group-selective agonists) (Table 1).
A number of agonists and antagonists for metabotropic glutamate receptors are highly selective for members within a group, and some pharmacological agents that distinguish receptors within groups have been described. Yet, this 'group' classification has been extensively used, allowing investigators to define pharmacological functions that are linked to different receptors within each group. This information, combined with data from immunocytochemistry and receptor knock-out animals, has allowed researchers to conceptualise the possible role of individual receptors. As more selective and systemically active pharmacological agents emerge, the usefulness of this group I, II, III classification will likely diminish. With this in mind, information in this chapter describes group specific compounds as 'non-selective'.
Function
Pharmacological studies with group specific agonists and antagonists have been used to suggest that in general group I metabotropic glutamate receptors increase the excitability of neurones, whereas group II and group III agonists appear to suppress neuronal excitability. The receptors and mechanisms by which group specific agonists enhance or suppress glutamatergic excitability is currently an active area of pharmacological research. Moreover, metabotropic glutamate receptors can also modulate the release and/or actions (directly or indirectly) of other neurotransmitters including GABA, purines, dopamine, 5-HT, and neuropeptides[1,4]. In the case of certain mGlu receptors (e.g. mGlu1, mGlu2), which have a peri- or extra-synaptic localisation[10], they are likely to be primarily recruited under conditions of enhanced or sustained glutamate release where the concentrations of synaptic glutamate is greatly increased (e.g. during conditions resulting from repetitive firing of neurones such as long-term potentiation or excitoxicity).
Species and splice variants
Metabotropic glutamate receptors belong to family 2.3 of the G protein-coupled receptors along with GABAB, pheromone, and Ca2+-sensing receptors[2]. Mammalian metabotropic glutamate receptors have been cloned from rat, mouse, and human species. These studies have shown very high (~95--99%) homology between species for each receptor. A number of splice variants have been shown to exist for most, but not all receptors (Table 2). Splice variants, generally involve changes in aa structure down-stream from the N-terminal domain. Thus, at least for pharmacological agents acting at the glutamate binding sites (agonists and antagonists), splice variants do not greatly alter the pharmacology of the receptor. However, as newer compounds are being discovered that might interact at sites distal from the glutamate binding region (e.g. non-competitive antagonists) this should not be assumed. Certain splice variants have been found (e.g. mGlu1(e)) in which a stop codon is introduced prior to TM1, producing a soluble secretable protein containing the glutamate binding region. The functional significance of this soluble receptor form is not known.
Additional receptors
Pharmacological data suggest the existence of other metabotropic glutamate receptors that are coupled to phospholipase D (refs. [3,7,11]). However, a molecular form of any of these receptors has not been identified. Pharmacological data also suggest the existence of a novel phospholipase C (PLC)-coupled metabotropic glutamate receptor which is activated by bromohomoibotenic acid [5].
Transduction mechanisms
Through their coupling to Gq (group I) or Gi/Go (groups II and III), metabotropic glutamate receptors are linked to a number of transduction mechanisms in addition to activation of PLC or inhibition of adenylate cyclase. These include stimulation of adenylate cyclase (group I), regulation of Ca2+ channels (groups I, II and III), and regulation of K+ channels (group I and II) [2,6,12-13]. In general, knowledge of the coupling of individual receptors to various transduction mechanisms is hampered by the lack of selective pharmacological agents.References
1. Anwyl R. (1999) Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev, 29 (1): 83-120. [PMID:9974152]
2. Bockaert J, Pin JP. (1999) Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J, 18 (7): 1723-9. [PMID:10202136]
3. Boss V, Conn PJ. (1992) Metabotropic excitatory amino acid receptor activation stimulates phospholipase D in hippocampal slices. J Neurochem, 59 (6): 2340-3. [PMID:1431912]
4. Cartmell J, Schoepp DD. (2000) Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem, 75 (3): 889-907. [PMID:10936169]
5. Chung DS, Winder DG, Conn PJ. (1994) 4-Bromohomoibotenic acid selectively activates a 1-aminocyclopentane-1S,3R-dicarboxylic acid-insensitive metabotropic glutamate receptor coupled to phosphoinositide hydrolysis in rat cortical slices. J Neurochem, 63 (1): 133-9. [PMID:8207423]
6. Conn PJ, Pin JP. (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol, 37: 205-37. [PMID:9131252]
7. Klein J, Iovino M, Vakil M, Shinozaki H, Löffelholz K. (1997) Ontogenetic and pharmacological studies on metabotropic glutamate receptors coupled to phospholipase D activation. Neuropharmacology, 36 (3): 305-11. [PMID:9175608]
8. Nakanishi S. (1992) Molecular diversity of glutamate receptors and implications for brain function. Science, 258: 597-603. [PMID:1329206]
9. O'Hara PJ, Sheppard PO, Thøgersen H, Venezia D, Haldeman BA, McGrane V, Houamed KM, Thomsen C, Gilbert TL, Mulvihill ER. (1993) The ligand-binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron, 11 (1): 41-52. [PMID:8338667]
10. Ottersen OP, Landsend AS. (1997) Organization of glutamate receptors at the synapse. Eur J Neurosci, 9 (11): 2219-24. [PMID:9464917]
11. Pellegrini-Giampietro DE, Torregrossa SA, Moroni F. (1996) Pharmacological characterization of metabotropic glutamate receptors coupled to phospholipase D in the rat hippocampus. Br J Pharmacol, 118 (4): 1035-43. [PMID:8799579]
12. Pin JP, De Colle C, Bessis AS, Acher F. (1999) New perspectives for the development of selective metabotropic glutamate receptor ligands. Eur J Pharmacol, 375 (1-3): 277-94. [PMID:10443583]
13. Pin JP, Duvoisin R. (1995) The metabotropic glutamate receptors: structure and functions. Neuropharmacology, 34 (1): 1-26. [PMID:7623957]
14. Schoepp DD, Conn PJ. (1993) Metabotropic glutamate receptors in brain function and pathology. Trends Pharmacol Sci, 14 (1): 13-20. [PMID:7680175]
15. Schoepp DD, Jane DE, Monn JA. (1999) Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology, 38 (10): 1431-76. [PMID:10530808]







