|
Compound class:
Synthetic organic
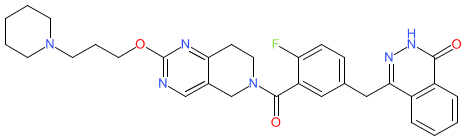
Comment: This compound is reported to exhibit dual functions as a poly ADP-ribose polymerase 1 (PARP1) inhibitor and cyclin-dependent kinase 12 (CDK12) inhibitor [1]. These two enzymes are important components of the DNA damage repair (DDR) response. CDK12 may be overexpressed in aggressive HER2-positive breast cancers. PARP inhibition is an established mechanism that is employed to disrupt DDR in cancer cells, particulary those with existing defects in homologous recombination such as BRCA-mutated breast cancers. Compound 9 was developed to investigate the anti-tumour potential of simultaneous disruption of the activities of these two molecular targets. It acts is a direct inhibitor of PARP1 and reduces expression of CDK12.
|
|
|||||||||||||||||||||||||||||||||||
| Selectivity at enzymes | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||