|
Compound class:
Synthetic organic
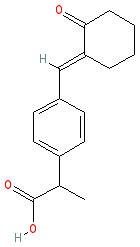
Comment: Pelubiprofen is a nonsteroidal anti-inflammatory type compound [6]. Structurally it is an aryl propionic acid derivative, like ibuprofen. The INN-defined agent is a racemic mixture of enantiomers. We show the structure without specified stereochemistry to represent the mixture. Pelubiprofen is described as a prodrug of 2-arylpropionic acid with modest selectivity for COX2 [3].
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Asami M, Shigeta A, Tanaka Y. (1996)
Disposition of CS-670, a novel nonsteroidal anit-inflammatory drug, and its metabolites in healthy human volunteers. Chirality, 8 (2): 207-13. [PMID:8857182] |
|
2. Asami M, Takasaki W, Iwabuchi H, Haruyama H, Wachi K, Terada A, Tanaka Y. (1995)
Stereospecific taurine conjugation of the trans-OH metabolite (active metabolite) of CS-670, a new 2-arylpropionic acid nonsteroidal anti-inflammatory drug, in dogs. Biol Pharm Bull, 18 (11): 1584-9. [PMID:8593485] |
|
3. Choi IA, Baek HJ, Cho CS, Lee YA, Chung WT, Park YE, Lee YJ, Park YB, Lee J, Lee SS et al.. (2014)
Comparison of the efficacy and safety profiles of a pelubiprofen versus celecoxib in patients with rheumatoid arthritis: a 6-week, multicenter, randomized, double-blind, phase III, non-inferiority clinical trial. BMC Musculoskelet Disord, 15: 375. [PMID:25403311] |
|
4. Kobayashi K, Abe C, Iizuka Y, Shiokawa Y. (1986)
Immunomodulation by RS-2131, a new non-steroidal antiinflammatory drug. Immunopharmacology, 12 (3): 213-20. [PMID:3546192] |
|
5. Shin JS, Baek SR, Sohn SI, Cho YW, Lee KT. (2011)
Anti-inflammatory effect of pelubiprofen, 2-[4-(oxocyclohexylidenemethyl)-phenyl]propionic acid, mediated by dual suppression of COX activity and LPS-induced inflammatory gene expression via NF-κB inactivation. J Cell Biochem, 112 (12): 3594-603. [PMID:21809372] |
|
6. Terada A, Naruto S, Wachi K, Tanaka S, Iizuka Y, Misaka E. (1984)
Synthesis and antiinflammatory activity of [(cycloalkylmethyl)phenyl]acetic acids and related compounds. J Med Chem, 27 (2): 212-6. [PMID:6607354] |