|
Synonyms: PF-04171327
Compound class:
Synthetic organic
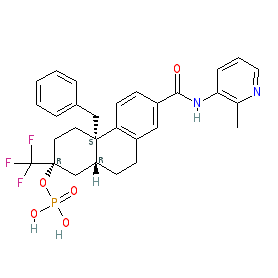
Comment: Fosdagrocorat (PF-04171327) is a glucocorticoid (GC) receptor partial agonist with anti-inflammatory potential. It is an example of a new class of GC substances termed dissociated agonists of the GC receptors (DAGR), that are being developed to improve the risk-benefit profile of GC drugs. DAGRs are designed to favour interaction of the GC receptor with protein partners rather than DNA, such that the transrepressive actions are enhanced over the transactivation effects. The hypothesis is that dissociating transrepression from transactivation will cause less undesirable effects on bone and glucose metabolism compared to current GC agonists such as prednisone (reviewed in [1]). Fosdagrocorat is example 2 in Pfizer's patent US8901310 [3]. It is a phosphate ester prodrug of dagrocorat (example 1 in US8901310).
Synthesis and structure-activity relationship (SAR) of another series of DAGRs (by Boehringer Ingelheim Pharmaceuticals) are reported in [2]. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Results of a Phase 2 clinical trial evaluating fosdagrocorat in patients with rheumatoid arthritis (RA) have been published [4], which report positive outcomes with regards to efficacy in improving RA signs and symptoms, with manageable adverse events. The efficacy of fosdagrocorat (10 and 15 mg/day) was equivalent to 10 mg/day prednisone, whereas its impact on biomarkers for bone formation and resorption, and plasma glucose were comparable to a lower prednisone dose of 5 mg/day. |