|
Synonyms: ATB 346 | ATB-346 | ATB-346, (S)- | ATB346
Compound class:
Synthetic organic
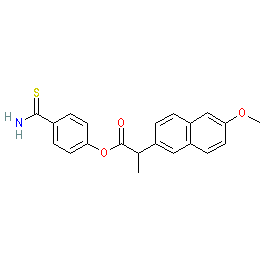
Comment: Otenaproxesul (ATB-346) is a novel, orally administered, gastrointestinal-sparing hydrogen sulfide (H2S)-releasing analgesic and anti-inflammatory drug in clinical trials for osteoarthritis [8]. Structurally it comprises an H2S-releasing moiety covalently linked to a NSAID (naproxen). H2S-releasing NSAIDs are in development for their potential analgesic, anti-inflammatory, cytoprotective and chemopreventative properties [2-3,5-6,9-10].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| A pivotal, Phase 2B dose-ranging, efficacy study is underway comparing three doses of ATB-346 to placebo in patients with osteoarthritis of the knee. Four Phase 1 and 2 clinical studies have been completed, including part 1 of the ongoing study. The first Phase 2B study (see NCT03291418) showed that GI toxicity with ATB-346 is markedly reduced (compared to naproxen) following 14 days of treatment, whilst producing the same magnitude of COX inhibition [8]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03291418 | To Compare the Gastrointestinal Safety of a 14-Day Oral Dosing Regimen of ATB-346 to Sodium Naproxen in Healthy Subjects | Phase 1/Phase 2 Interventional | Antibe Therapeutics Inc. | ||