|
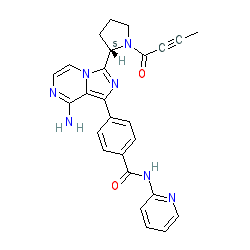
Synonyms: ACP-196 | Calquence® | Example 6 [US20140155385 A1] [2]
acalabrutinib is an approved drug (FDA (2017), EMA (2020))
Compound class:
Synthetic organic
Comment: Acalabrutinib is an orally available second-generation, selective and irreversible inhibitor of Bruton tyrosine kinase (BTK) [6], being investigated for its potential antineoplastic activity (in multiple haematologic malignancies and solid tumours), as well as a potential therapy for rheumatoid arthritis. Acalabrutinib covalently bonds to Cysteine-481 in BTK.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| Pharmacological inhibition of BTK prevents the activation of B cells and BTK-mediated activation of downstream survival pathways. This leads to inhibition of the growth of malignant B cells overexpressing BTK. |
| Immunopharmacology Disease | |||
| Disease | X-Refs | Comment | References |
| Rheumatoid arthritis |
Disease Ontology:
DOID:7148 OMIM: 180300 |
Phase 2 clinical trial in RA completed (NCT02387762) | |
| B-cell chronic lymphocytic leukemia |
OMIM:
151400 Orphanet: ORPHA67038 |
Phase 3 clinical candidate for CLL. | |
| Mantle cell lymphoma |
Disease Ontology:
DOID:0050746 Orphanet: ORPHA52416 |
Approved drug for MCL patients who have received at least one prior therapy. | 7 |