|
Synonyms: Abitrexate® | amethopterin | Nordimet® | Rasuvo®
methotrexate is an approved drug (FDA (1953), EMA (2016))
Compound class:
Synthetic organic
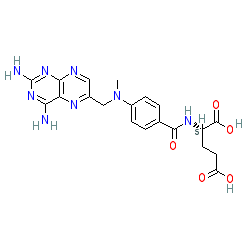
Comment: Methotrexate is a folate (folic acid) analogue that is classified as an antimetabolite and antifolate drug, and a disease-modifying anti-rheumatic drug (DMARD). It is used clinically for its antiproliferative and anti-inflammatory effects. The primary action of methotrexate is inhibition of the enzyme dihydrofolate reductase (DHFR). DHFR is essential for the production of precursors that are required for de novo purine synthesis.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: methotrexate |
|
|||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| Methotrexate is a DMARD prescribed for rheumatoid arthritis, psoriatic arthritis and vasculitis. In inflammation its mode of action relates to its ability to inhibit folate pathway enzymes, including aminoimidazole carboxamide adenosine ribonucleotide transformylase (ATIC; P31939). In vivo conversion of methotrexate to methotrexate-polyglutamates is required for ATIC inhibition [2]. ATIC inhibition leads to intracellular accumulation of its substrate aminoimidazole carboxamide adenosine ribonucleotide (AICAR), increased adenosine release and modulation of immune cell (neutrophils, natural killer cells, monocytes/macrophages and T cells) function, effects which ultimately produce a potent anti-inflammatory effect [1,4]. Key inflammatory mediators such as IL-17, IL-22, IL-23 and CCL20 are downregulated by methotrexate. Low-dose methotrexate was evaluated to determine if inflammation inhibition could provide an effective approach for the secondary prevention of atherosclerotic events (heart attack, stroke, and cardiovascular death) in the phase 3 Cardiovascular Inflammation Reduction Trial (CIRT; NCT01594333) [3]. Analysis of results from CIRT concluded that methotrexate was no more effective than placebo in patients with stable atherosclerosis; it did not reduce the number of cardiovascular events, or reduce inflammation markers such as IL-1β, IL-6 and C-reactive protein, relative to placebo-treated controls [6]. |
| Immunopharmacology Disease | |||
| Disease | X-Refs | Comment | References |
| Vasculitis |
Disease Ontology:
DOID:865 |
Approved drug for vasculitis. | |
| Rheumatoid arthritis |
Disease Ontology:
DOID:7148 OMIM: 180300 |
Approved drug for RA. | |
| Psoriatic arthritis |
Disease Ontology:
DOID:9008 |
Approved drug for PsA. | |
| Juvenile idiopathic arthritis | Oral solution of methotrexate approved for JIA in pediatric pateints. | ||