|
Synonyms: HepFlush®
heparin is an approved drug (FDA (1939))
Compound class:
Metabolite
Comment: Heparin is an endogenous secretory glycosaminoglycan (GAG) that is released predominantly from connective tissue mast cells. Isolated heparin is used clinically as an anti-coagulant. Structurally, heparins (generally known as unfractionated heparin) are a heterogeneous group of anionic, sulfated GAGs. Commercial preparations usually vary in size from 12-15 kDa, while a low molecular weight (fractionated) versions are also available. Database entries may specify the sodium salt forms. SARS-CoV-2: There is strong experimental evidence which shows that heparin interacts directly with GAG-binding motifs on the Spike glycoprotein of SARS-CoV-2 [ 3], and that inhibiting this interaction can reduce SARS-CoV-2 infection of host cells [ 6, 8]. These findings suggest that heparin may offer repurposing potential as a prophylactic COVID-19 therapeutic [ 2], in addition to its benefit as a treatment for COVID-19-related thrombotic complications.
|
|
2D Structure
|
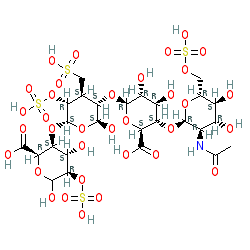
|
Physico-chemical Properties
|
|
|
Hydrogen bond acceptors
|
35
|
|
Hydrogen bond donors
|
14
|
|
Rotatable bonds
|
19
|
|
Topological polar surface area
|
588.61
|
|
Molecular weight
|
1039.04
|
|
XLogP
|
-11.46
|
|
No. Lipinski's rules broken
|
3
|
|
SMILES / InChI / InChIKey
|
|
|
Canonical SMILES
|
CC(=O)NC1C(OC2C(OC(C(C2O)O)OC2C(O)OC(C(C2CS(=O)(=O)O)OS(=O)(=O)O)OC2C(OC(C(C2O)OS(=O)(=O)O)O)C(=O)O)C(=O)O)OC(C(C1O)O)COS(=O)(=O)O
|
|
Isomeric SMILES
|
CC(=O)N[C@H]1[C@@H](O[C@@H]2[C@H](O[C@H]([C@@H]([C@H]2O)O)O[C@@H]2[C@@H](O)O[C@@H]([C@@H]([C@H]2CS(=O)(=O)O)OS(=O)(=O)O)O[C@@H]2[C@@H](OC([C@@H]([C@H]2O)OS(=O)(=O)O)O)C(=O)O)C(=O)O)O[C@@H]([C@H]([C@@H]1O)O)COS(=O)(=O)O
|
|
InChI
|
InChI=1S/C26H41NO34S4/c1-4(28)27-7-9(30)8(29)6(2-52-63(43,44)45)53-24(7)56-15-10(31)11(32)25(58-19(15)21(36)37)55-13-5(3-62(40,41)42)14(60-64(46,47)48)26(59-22(13)38)57-16-12(33)17(61-65(49,50)51)23(39)54-18(16)20(34)35/h5-19,22-26,29-33,38-39H,2-3H2,1H3,(H,27,28)(H,34,35)(H,36,37)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)/t5-,6+,7+,8+,9+,10+,11+,12-,13-,14+,15-,16-,17+,18+,19-,22-,23?,24+,25+,26-/m0/s1
|
|
InChI Key
|
ZFGMDIBRIDKWMY-PASTXAENSA-N
|
|
|









