GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
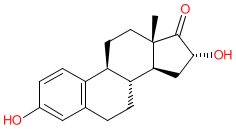
Synonyms: 16α-OHE1 | 16-hydroxyestrone | 16alpha-hydroxyestrone | 16alpha-OHE1
Compound class:
Synthetic organic
Comment: 16α-hydroxyestrone is an estrogenic metabolite that is a 16-hydroxylation product generated during the catalytic breakdown of estrone by cytochrome P450 enzymes in the liver (principally CYP3A4 and 3A5).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Fishman J, Martucci C. (1980)
Biological properties of 16 alpha-hydroxyestrone: implications in estrogen physiology and pathophysiology. J Clin Endocrinol Metab, 51 (3): 611-5. [PMID:7190977] |
|
2. Fishman J, Schneider J, Hershcope RJ, Bradlow HL. (1984)
Increased estrogen-16 alpha-hydroxylase activity in women with breast and endometrial cancer. J Steroid Biochem, 20 (4B): 1077-81. [PMID:6727352] |
|
3. Martucci C, Fishman J. (1977)
Direction of estradiol metabolism as a control of its hormonal action--uterotrophic activity of estradiol metabolites. Endocrinology, 101 (6): 1709-15. [PMID:590186] |
|
4. Schneider J, Kinne D, Fracchia A, Pierce V, Anderson KE, Bradlow HL, Fishman J. (1982)
Abnormal oxidative metabolism of estradiol in women with breast cancer. Proc Natl Acad Sci U S A, 79 (9): 3047-51. [PMID:6953448] |
|
5. Swaneck GE, Fishman J. (1988)
Covalent binding of the endogenous estrogen 16 alpha-hydroxyestrone to estradiol receptor in human breast cancer cells: characterization and intranuclear localization. Proc Natl Acad Sci U S A, 85 (21): 7831-5. [PMID:3186693] |